In 2011, the U.S. Food & Drug Administration (FDA) published their Guidance for Industry – Process Validation: General Principles and Practices. A PharmManufacturing.com article, A Framework for Technology Transfer to Satisfy the Requirements of the New Process Validation Guidance: Part 2 shared the impact on the Life Sciences industry:
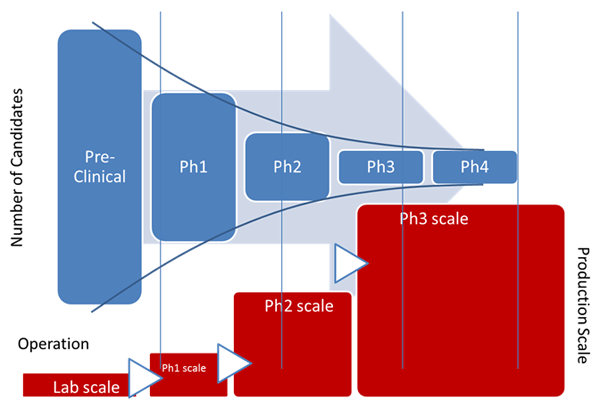
In the life of any drug product, the technology transfer of a process is a complex matter, made more complicated by the new definition of the Process Validation (PV) guidance issued by FDA in January 2011.

Zuwei Jin
Senior Life Sciences Consultant
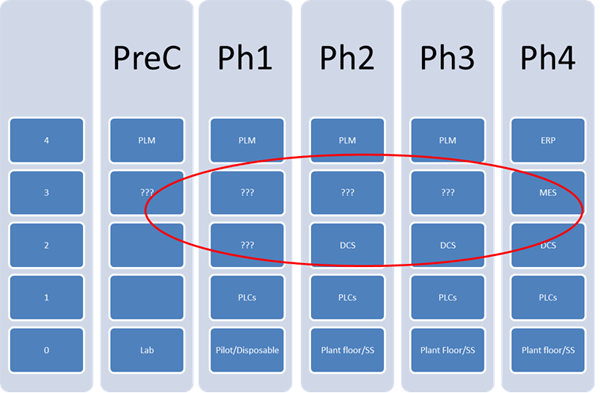
Zuwei believes that early adoption of MES, as defined in ISA-95 (S95), a standard addressing the enterprise control system interface, and ISA-88 (S88), a standard addressing batch process control, provide a framework for implementing not only the technical but also the regulatory and business processes required to support the tech transfer and expansion throughout the drug development cycle. It is a key element in introducing a scalable, modular, hardware independent platform for drug development and data management.
He feels that early adoption of an MES platform leads to consistent engineering and business practices throughout the drug development cycle while MES and ISA-88 allow processes to be defined on unified framework. This platform also helps with equipment commissioning and allows more effective communication between the end-users, suppliers, and engineering companies. The practice benefits the technology transfer and plant expansion from phase to phase tremendously as the result of using the same framework. MES bridges the gap between level 2 and 4 throughout the drug development and should be introduced as early as one can.
As part of the design and engineering effort, incorporation of the MES software platform into the framework leads to a consistent engineering and documentation practice in equipment qualification and commissioning. Even more importantly, the practice benefits the next phase and expansion tremendously as the technology transfer can be carried out on the same framework.
Zuwei notes that it makes for a much easier process of transferring technology from site to site and/or country to country for similar reasons. Adopting the MES platform and following the S88 standard helps improve speed to the market (better project time), reinforce regulatory compliance (better project quality), enforce consistent technical and business practice (smoother workflow), and increase efficiency and cost effectiveness (better labor utilization).
Many pharmaceutical and biotech manufacturers have adopted this approach, particularly in their new facilities and have improved their technology transfer practice through the introduction of the MES and S95 standards. Coupled with an MES platform in the early phase, this combination is particularly attractive for Greenfield projects, due to the importance of compliance expertise and time to market. This approach will likely receive even more attention in Asia where many more Greenfield projects exist and the demand for speed to market and regulatory compliance are great.
You can connect and interact with other pharmaceutical, biotech, and manufacturing execution system experts in the Life Sciences and Operations Management tracks of the Emerson Exchange 365 community.