In this podcast, Emerson’s Jorge Costa and Bruce Greenwald join Jim Cahill to discuss the application of PAT technologies not only within the Life Sciences but across many of the process and hybrid manufacturing industries.
biotech
Achieving Data Integrity, Quality & Compliance in Manufacturing
In a recent webinar, Achieve Data Integrity, Quality & Compliance Across Your Organization, Emerson’s Michalle Adkins and Hilary Mills-Baker share how data integrity means complete, consistent, accurate data throughout its lifecycle.
Digital Solutions for Process Development, Tech Transfer and Manufacturing
Emerson’s Michalle Adkins and AspenTech’s Chuck Miller team up to present Robust PAT Solutions to Support Process Development, Tech Transfer and Manufacturing at IFPAC 2023.
Better Treatment—For the Earth, and its People
It can be hard to keep up with the many rapid changes occurring in the life sciences industry these days. In an effort to embrace improved speed to market, sustainability, and quality, life sciences manufacturers are employing a wide range of software and technologies...
Improving Digital Maturity is Key to Life Sciences Success
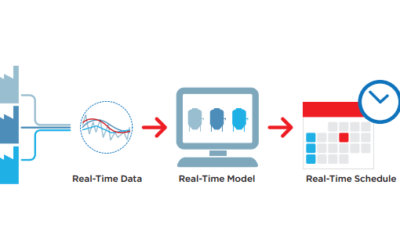
In just the last 10 years, biopharmaceutical supply chains have changed dramatically. The single-source manufacturing model is becoming much rarer, making way for multi-enterprise manufacturing collaborations and partnerships to better meet the changing needs of the...
Leverage Life Sciences Software to Reduce Time to Market
The days of maintaining paper records across the life sciences development and production pipeline are gone. Today’s regulatory requirements, not to mention the complexity of new treatments, requires organization that can only be provided electronically. However,...
Module Type Package Unlocks the Flexibility Driving Pharma Innovation
There can be little question that Industry 4.0 technologies are reshaping the way life sciences companies operate. In the wake of the rapid development of vaccines during the COVID-19 pandemic, more facilities than ever are being “born digital.” Some of these...
BioPhorum Cell and Gene Therapy Personas and User Stories
The BioPhorum CGT Personas and User Stories toolkit details the needs of all the key players involved in end-to-end cell and gene therapy (CGT) processes. It can be used by anyone who wishes to better understand how IT systems can support the manufacture and delivery of CGTs.
Is the Biotech Industry Growing?
For biotech industries, Emerson offers fundamental automation solutions with quicker uptimes and more consistent batch quality.
Improving Equipment Reliability for Life Sciences Manufacturers
Reliability of assets used in the manufacturing process is paramount for safe, efficient and effective operations. Unplanned asset failures, improper asset performance, poor maintenance planning, and untimely follow-through can cause shutdowns, under-utilized production equipment, deviations, or material/product losses.
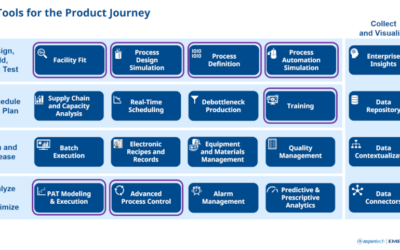
Trends in Process Analytical Technology and Quality by Design
At the recent conference for pharmaceutical and biotech manufacturers, Interphex 2012, Pharmaceutical Manufacturing magazine Editor in Chief Agnes Shanley discussed trends in the industry's use of process analytical technology (PAT) and quality by design (QbD) with...
Continuous Process Verification per FDA Process Validation Guidance
It was just about a year ago that the U.S. Food and Drug Administration published their Guidance for Industry - Process Validation: General Principles and Practices. I caught up with Emerson's Heather Schwalje, a senior consultant on the Life Sciences industry team....
Keep Up to Date With the Latest News and Updates
Follow Us
We invite you to follow us on Facebook, LinkedIn, Twitter and YouTube to stay up to date on the latest news, events and innovations that will help you face and solve your toughest challenges.
Do you want to reuse or translate content?
Just post a link to the entry and send us a quick note so we can share your work. Thank you very much.
Our Global Community
Emerson Exchange 365
The opinions expressed here are the personal opinions of the authors. Content published here is not read or approved by Emerson before it is posted and does not necessarily represent the views and opinions of Emerson.