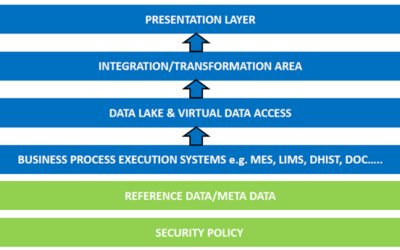
Author: Jonathan Lustri Almost all Life Sciences manufacturing execution system (MES) projects include an interface to SAP, the enterprise resource management system. This interface is strategic in that this is how the business and production are tied together. It...
Jonathan Lustri
Best Practices for Implementing a Process Control Driven MES System
Author: Jonathan Lustri I have previously been interviewed and written about a process control and MES [Manufacturing Execution Systems] architecture where the batch control logic within the process control system is the single procedural engine. It drives procedural...
Successfully Implementing Process Analytical Technology
It's been well more than a decade since the U.S. Food & Drug Administration (FDA) announced a Process Analytical Technology (PAT) approach for pharmaceutical manufacturers in their Guidance for Industry PAT — A Framework for Innovative Pharmaceutical Development,...
Challenge of Designing PCS-Driven MES Architectures for a Greenfield Facility
Author: Jonathan Lustri I have previously written about a design strategy where the process control system (PCS) is the primary system driving all procedural batch activity within a pharmaceutical process. In this architecture, the PCS ISA-88 procedural model must...
Best Practices Specifying MES Workflows for Life Sciences Projects
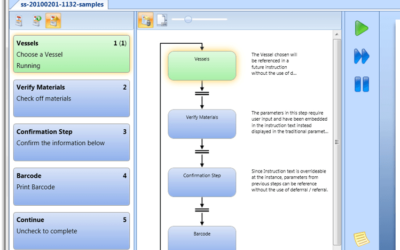
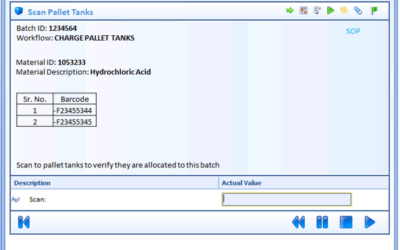
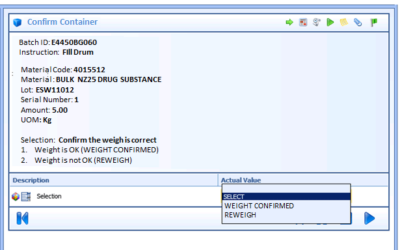
Author: Jonathan Lustri Manufacturing Execution System (MES) Life Sciences projects can be costly and be a challenge to keep in control. The biggest contributor to these challenges are the defining and design of manufacturing workflows. A Workflow is an MES procedural...
Deploying Syncade Manufacturing Execution System in a Non-English Language
Author: Jonathan Lustri I recently worked on a Syncade manufacturing execution system (MES) project where the system was deployed in Danish. Emerson executes all its projects with a global team and this one was no different. Our customer was Danish and we had team...
Overcoming Pharmaceutical Continuous Manufacturing Challenges
Why is there a movement in the pharmaceutical and biotech manufacturing industries to consider continuous manufacturing over traditional batch manufacturing processes? A 2012 U.S. Food & Drug Administration presentation, FDA Perspective on Continuous...
Is Process Analytical Technology Rocket Science?
More than a decade ago, the U.S. Food and Drug Administration published, Guidance for Industry PAT — A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance. In this document, the FDA explained: The scientific, risk-based framework...
Optimizing Batch Process Automation Design in MES and DCS
For pharmaceutical and biotech manufacturers, the flow and capture of information is as important as the flow of the manufacturing process in readying products to release for sale. In a Pharmaceutical Technology magazine article, Connecting MES to Process Control,...
Conference Room Pilots to Generate Involvement in MES Projects
Author: Jonathan Lustri As a Life Sciences consultant, I get involved in many different aspects of MES [manufacturing execution system] projects. In a recent project, I helped define the project execution plan. I knew Emerson's biotech customer was highly concerned...
Register Now for the Emerson Exchange Denver Conference
Early Bird registration opens today for the October 12-16, 2015 Emerson Exchange conference in Denver, Colorado. You'll save $200 USD by registering by August 15. You can see the full list of accepted presentation abstracts here. I'll highlight several more of the...
Advancing Process Intelligence and Analytics in Life Sciences
In a recent post, Collaborating for End-to-End Supply Chain Improvements, we highlighted a Life Sciences symposium where challenges to business improvements were discussed as inputs for technology and solution innovation to address these challenges. One of the...
Keep Up to Date With the Latest News and Updates
Follow Us
We invite you to follow us on Facebook, LinkedIn, Twitter and YouTube to stay up to date on the latest news, events and innovations that will help you face and solve your toughest challenges.
Do you want to reuse or translate content?
Just post a link to the entry and send us a quick note so we can share your work. Thank you very much.
Our Global Community
Emerson Exchange 365
The opinions expressed here are the personal opinions of the authors. Content published here is not read or approved by Emerson before it is posted and does not necessarily represent the views and opinions of Emerson.