Accelerating vaccine development requires unprecedented flexibility in managing data. With increasing integration between product lifecycle management systems and software—like distributed control systems (DCS) and manufacturing execution systems (MES)—data, recipes, and processes can be digitally altered, shared, and used to scale across the lifecycle.
Syncade
Improving Logical Changeover Production Readiness in the Life Sciences
Technology has continued to advance and provide ways to assist pharmaceutical & biopharmaceutical manufacturers with production readiness. Improper set up and qualification of automation and information management production applications cause start-up and product changeover delays, as well as slow performance during production runs.
Emerson Technologies Supporting Fast COVID-19 Vaccine Development and Rollout
In a Pharmaceutical Processing World article, Emerson’s Ben Arriola and Zach Blum discuss technologies advancing vaccine development. It’s safe to say that there has never been a pharmaceutical effort so huge, fast, and successful as developing multiple vaccines for...
Making Medicine Personal with Flexible Automation Solutions
Personalized medicine manufacturers are rapidly changing their manufacturing platforms to provide a new scale of production. In the Emerson Exchange Virtual Series, life sciences experts held an open Q&A on the trajectory of cell and gene therapies, and how a...
Digital Transformation Helps Moderna Safely Deliver COVID-19 Vaccine in Record Time
A common theme across the Emerson Exchange Virtual Series has been the way software and digital technologies have helped us all deal with an unexpected and unprecedented pandemic. Wherever there is adversity, there are also innovators who step up to the challenge of...
Digitalizing and Automating Review by Exception
In an Intech article, Three strategies help life sciences companies implement more successful review by exception, Emerson’s Emilee Cook shares ways for these manufacturers to improve this workflow.
Digital Tools More Quickly Deliver Treatments to Patients
Imagine driving the wrong direction for hundreds of miles after a wrong turn, simply because you didn’t notice missing the exit. Overlooked deviations can cause a lot of frustration, whether during a drive or while executing a batch of pharmaceuticals. Detecting and...
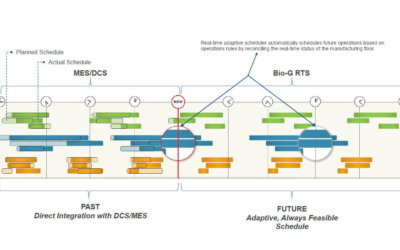
Real-Time Adaptive Scheduling in Life Science Manufacturing
At the Biomanufacturing World Summit, Emerson’s Ron Rossbach presented on using digitalization and data integration to help drive operational efficiency. A key enabling technology for these performance gains is in real-time adaptive scheduling. This technology combines finite scheduling with dynamic updates from existing shop-floor execution systems to optimize operations. In 2018 Emerson acquired BioG, the leading provider of adaptive scheduling software, which is now being adopted across the industry.
Manufacturing Execution Systems in Drug Research and Development
The U.S. Food & Drug Administration (FDA) outlines the drug development process in five steps: Discovery and development Preclinical research Clinical trials FDA review FDA post-market safety monitoring I caught up with Emerson's Zuwei Jin whom you may recall from...
Tracking Cell Therapy Chain of Identity
In this 2:25 YouTube video, Address Cell Therapy Batch Production Challenges with Syncade, Emerson’s Michalle Adkins shares how operations management technology plays an important role in addressing these challenges.
Best Practices for Implementing a Process Control Driven MES System
Author: Jonathan Lustri I have previously been interviewed and written about a process control and MES [Manufacturing Execution Systems] architecture where the batch control logic within the process control system is the single procedural engine. It drives procedural...
Moving to Electronic Batch Records
Yesterday we highlighted advancements in exception management technology for pharmaceutical and biopharmaceutical manufacturers. Exception management is one element in an electronic batch record (EBR). Other elements for the EBR which also have high data integrity...
Keep Up to Date With the Latest News and Updates
Follow Us
We invite you to follow us on Facebook, LinkedIn, Twitter and YouTube to stay up to date on the latest news, events and innovations that will help you face and solve your toughest challenges.
Do you want to reuse or translate content?
Just post a link to the entry and send us a quick note so we can share your work. Thank you very much.
Our Global Community
Emerson Exchange 365
The opinions expressed here are the personal opinions of the authors. Content published here is not read or approved by Emerson before it is posted and does not necessarily represent the views and opinions of Emerson.