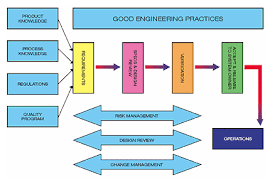
Highly regulated industries, such as pharmaceutical and biotech manufacturing, face a myriad of compliance issues, which vary by region of the world and continuously involve. Emerson's Heather Schwalje presented Regulatory Drivers- GMPs 21st Century to Life Science...
International Conference on Harmonization
ASTM E2500-Early, Targeted Testing Leads to Faster Implementations
Last week, I highlighted Emerson's Heather Schwalje thoughts on Continuous Process Verification per U.S. Food & Drug Administration (FDA) guidelines. In a comment, Pharmaceutical Manufacturing magazine senior editor, Paul Thomas, highlighted a great article, A...
Keep Up to Date With the Latest News and Updates
Follow Us
We invite you to follow us on Facebook, LinkedIn, Twitter and YouTube to stay up to date on the latest news, events and innovations that will help you face and solve your toughest challenges.
Do you want to reuse or translate content?
Just post a link to the entry and send us a quick note so we can share your work. Thank you very much.
Our Global Community
Emerson Exchange 365
The opinions expressed here are the personal opinions of the authors. Content published here is not read or approved by Emerson before it is posted and does not necessarily represent the views and opinions of Emerson.