Last week, the 2nd International Summit on GMP, GCP & Quality Control was held in Chicago, Illinois USA. Emerson's Heather Schwalje, a senior Life Sciences consultant, presented Moving beyond part 11; Quality assurance considerations for translating Current Good...
Heather Schwalje
Managing Asset Performance and Collaborating in the Life Science Industry
In two very different industries, I have some updates to share from Emerson consultants. On the reliability front, when we last checked in with Emerson's Richard Barnes, a Senior Asset Optimization Consultant, he was at a refinery located on the Black Sea, assisting...
Good Manufacturing Practice Background and Overview
Highly regulated industries, such as pharmaceutical and biotech manufacturing, face a myriad of compliance issues, which vary by region of the world and continuously involve. Emerson's Heather Schwalje presented Regulatory Drivers- GMPs 21st Century to Life Science...
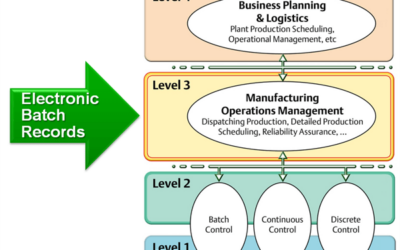
CPhI Pharma Evolution Guest Post-Electronic Batch Records: Are Your Systems Ready for Inspection?
I wanted to share my guest post published at the CPhI Pharma Evolution website. There are some great comments, so join in if the post sparks some ideas. Stringent inspection-readiness policies are common with pharmaceutical and biotech manufacturers. From a...
Keep Up to Date With the Latest News and Updates
Follow Us
We invite you to follow us on Facebook, LinkedIn, Twitter and YouTube to stay up to date on the latest news, events and innovations that will help you face and solve your toughest challenges.
Do you want to reuse or translate content?
Just post a link to the entry and send us a quick note so we can share your work. Thank you very much.
Our Global Community
Emerson Exchange 365
The opinions expressed here are the personal opinions of the authors. Content published here is not read or approved by Emerson before it is posted and does not necessarily represent the views and opinions of Emerson.