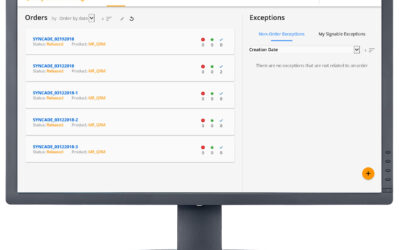
Exceptions in batch manufacturing processes are the deviations that occur outside the prescribed specifications. For pharmaceutical and biopharmaceutical manufacturers, quality and manufacturing personnel must review these exceptions. Traditionally, this quality...
exception reporting
Process Control with Wireless Devices
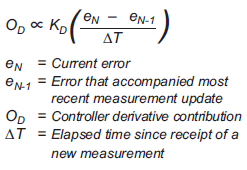
Emerson's Chris Womack discusses process control with IEC 62591 wireless devices in today's guest post. Using wireless devices in process control still isn't that common, but it's going to be. With its quick and easy installation, wireless technology is often used for...
Keep Up to Date With the Latest News and Updates
Follow Us
We invite you to follow us on Facebook, LinkedIn, Twitter and YouTube to stay up to date on the latest news, events and innovations that will help you face and solve your toughest challenges.
Do you want to reuse or translate content?
Just post a link to the entry and send us a quick note so we can share your work. Thank you very much.
Our Global Community
Emerson Exchange 365
The opinions expressed here are the personal opinions of the authors. Content published here is not read or approved by Emerson before it is posted and does not necessarily represent the views and opinions of Emerson.