Medicines produced by pharmaceutical and biotech manufacturers are manufactured in many ways. One particular type of treatment, cell therapy, is defined as: …the transplantation of human or animal cells to replace or repair damaged tissue. From a manufacturing...
manufacturing execution system
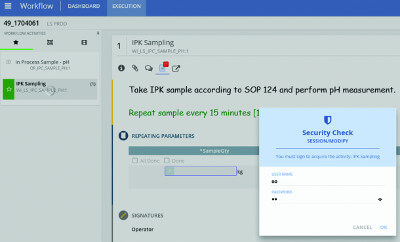
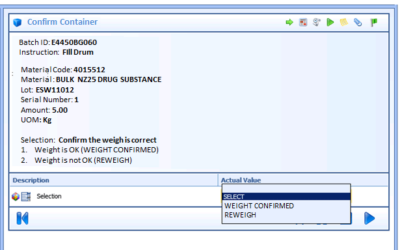
Improving Batch Manufacturing Workflows and Recordkeeping
The pharmaceutical and biotechnology manufacturing industries are challenged with errors in manual entries in the production process, getting batches right the first time, reaching final signoff for batch release, and managing the data accumulated throughout the...
Challenge of Designing PCS-Driven MES Architectures for a Greenfield Facility
Author: Jonathan Lustri I have previously written about a design strategy where the process control system (PCS) is the primary system driving all procedural batch activity within a pharmaceutical process. In this architecture, the PCS ISA-88 procedural model must...
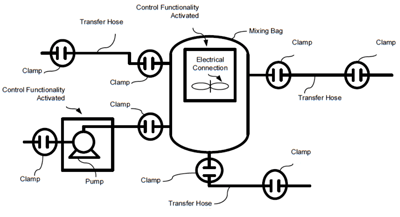
Automating Bioprocess Single-Use Systems
For those not in the pharmaceutical and biotech manufacturing industries, if you ever visit one of these facilities you'll be struck by the clean, shiny stainless steel vessels and piping. Given the importance of hygiene in the production of medicines, most processes...
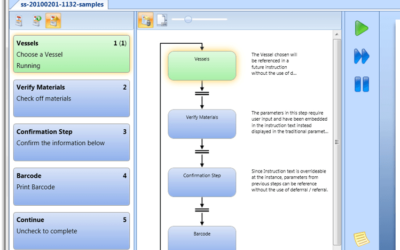
Best Practices Specifying MES Workflows for Life Sciences Projects
Author: Jonathan Lustri Manufacturing Execution System (MES) Life Sciences projects can be costly and be a challenge to keep in control. The biggest contributor to these challenges are the defining and design of manufacturing workflows. A Workflow is an MES procedural...
Integrating Single Use Equipment More Easily with Control and Manufacturing Execution Systems
One of the trends in the biopharmaceutical industries that have taken root over the past decade and a half is the use of single-use, disposable equipment in the manufacturing process. Prior to their use, these facilities relied on inflexible, hard-piped equipment. An...
Deploying Syncade Manufacturing Execution System in a Non-English Language
Author: Jonathan Lustri I recently worked on a Syncade manufacturing execution system (MES) project where the system was deployed in Danish. Emerson executes all its projects with a global team and this one was no different. Our customer was Danish and we had team...
Niche-Buster Medicine Information Solutions
Author: Michalle Adkins We are seeing a trend in the life sciences industry moving away from blockbusters to niche busters, population based medicines and even to the extreme of personalized medicines. The Emerson Life Sciences consulting team recently had the...
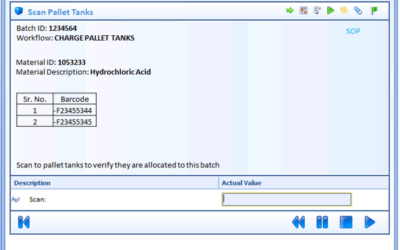
Optimizing Batch Process Automation Design in MES and DCS
For pharmaceutical and biotech manufacturers, the flow and capture of information is as important as the flow of the manufacturing process in readying products to release for sale. In a Pharmaceutical Technology magazine article, Connecting MES to Process Control,...
Data Integrity for Compliance, Audit Readiness and Performance
Pharmaceutical and biotech manufacturers have stringent regulatory compliance requirements. These requirements must balance with the need for improvement and optimization to run safely, efficiently, profitably and in line with the business and quality objectives. In a...
Conference Room Pilots to Generate Involvement in MES Projects
Author: Jonathan Lustri As a Life Sciences consultant, I get involved in many different aspects of MES [manufacturing execution system] projects. In a recent project, I helped define the project execution plan. I knew Emerson's biotech customer was highly concerned...
Reducing Communications Problems during Shift Changeovers
Sources of unplanned downtime can be many including equipment failures and human error. For downtime resulting from operator interactions, one major source of errors is the communications breakdown that sometimes happens during a shift change. If not told or seen in...
Keep Up to Date With the Latest News and Updates
Follow Us
We invite you to follow us on Facebook, LinkedIn, Twitter and YouTube to stay up to date on the latest news, events and innovations that will help you face and solve your toughest challenges.
Do you want to reuse or translate content?
Just post a link to the entry and send us a quick note so we can share your work. Thank you very much.
Our Global Community
Emerson Exchange 365
The opinions expressed here are the personal opinions of the authors. Content published here is not read or approved by Emerson before it is posted and does not necessarily represent the views and opinions of Emerson.