A Pharmaceutical Manufacturing magazine study revealed that pharmaceutical and biotech manufacturers were challenged to achieve batch after batch repeatability.
 I came across a recently published whitepaper, Consistency and Repeatability Through Accurate Measurements. Emerson’s Michalle Adkins played a role in its contents. The whitepaper highlights:
I came across a recently published whitepaper, Consistency and Repeatability Through Accurate Measurements. Emerson’s Michalle Adkins played a role in its contents. The whitepaper highlights:
…how reliable, accurate instrumentation allows automation to support quality and productivity strategies such as the process analytical technology (PAT) initiative, continued process verification (CPV), and the ICH Q10 Pharmaceutical Quality System.
The efficiency of a manufacturing process, as defined by in-spec production per unit time and/or cost, is related to the accuracy and repeatability of the measurement instrumentation.
Accurate and repeatable basic process measurements such as temperature, pressure and flow support the control necessary to achieve tight specifications for higher-level parameters such as pH, conductivity and dissolved oxygen (DO), as well as product composition, structure and efficacy.
The whitepaper describes the challenges controlling bioreactors used for manufacturing products such as monoclonal antibodies. Measurements in these hygienic and sanitary applications include:
…physical variables (temperature, pressure, power input, turbidity, and viscosity), biological variables (content of proteins, DNA) and chemical variables (pH value, O2, CO2, redox potential) of the process medium, as well as O2, CO2 and CH4 in the exhaust gas.
Deviations in pH, temperature or dissolved oxygen beyond narrow tolerance ranges can affect the cell’s growth and therefore also yield of the subject protein, as well as the protein structure.
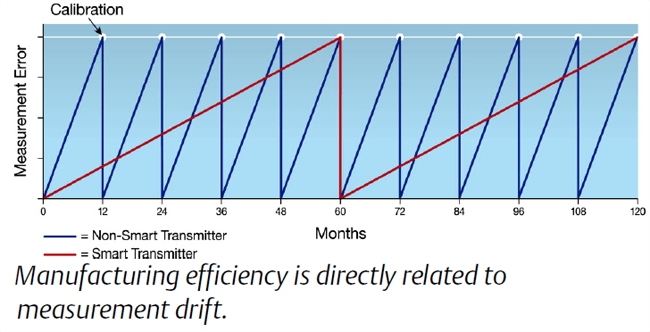
Smart measurement devices with embedded diagnostics can help significantly reduce the drift over time before recalibration procedures are required.
Another example of the importance of accurate and repeatable measurement instrumentation cited in the whitepaper is with tangential flow filtration (TFF) processes. Accurately controlling the crossflow and filtrate flow helps:
…to prevent membrane fouling, thus allowing a greater volume of product to be processed in the least possible time. Both rise together, and poor control leads to membrane fouling, extended filtering time and lost product or yield. Product that remains on the membrane cannot be recovered.
Effective implementations of the U.S. Food and Drug Administration’s PAT, CPV and ICH Q10 guidelines require:
…consistently collected and easily accessible data. Control system data historian and electronic batch record systems can support this functionality only if they record accurate process data from reliable instrumentation.
Read the whitepaper for more on deviation monitoring and the validation of changes and batch repeatability. When specifying measurement devices to meet these guidelines:
Seek temperature, pressure and flow transmitters that not only meet accuracy specifications, but also are certified to maintain accuracy for extended periods of time (stable) and, where appropriate, are equipped with self-diagnostics to alert operators they need calibration or maintenance. To ease installation and reduce paperwork, select instrumentation that is factory calibrated, documented and certified for compliance.
You can connect with measurement instrumentation and industry experts in the Flow, Pressure, Temperature and Life Sciences groups in the Emerson Exchange 365 community.