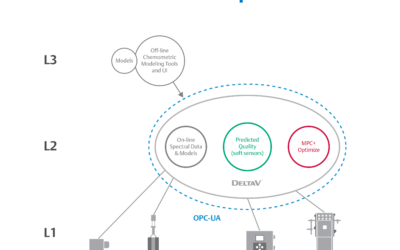
Traditional manufacturing practices have served the biopharmaceutical industry well for years, but times are changing and a need for speed to market in life sciences while reducing manufacturing costs is putting a focus on enabling real-time release (RTR)....
GMP
Improve Speed to Market with Closed-Loop Process Control using Spectral PAT
We have witnessed an incredible change the last few years in how life sciences manufacturers deliver treatments. Today’s focus is on fast results, and that means products patients rely on cannot sit on shelves for weeks or months waiting on quality validation. In a...
Good Manufacturing Practice Background and Overview
Highly regulated industries, such as pharmaceutical and biotech manufacturing, face a myriad of compliance issues, which vary by region of the world and continuously involve. Emerson's Heather Schwalje presented Regulatory Drivers- GMPs 21st Century to Life Science...
Managing the Pharmaceutical and Biotech Technology Transfer Process
In a Research and Markets report, Technology Transfer Strategies - A Guide to Maximizing Returns Within the Pharmaceutical and Biopharmaceutical Industries, the authors note: The efficient and effective transfer of new technologies is widely recognized as being a key...
Keep Up to Date With the Latest News and Updates
Follow Us
We invite you to follow us on Facebook, LinkedIn, Twitter and YouTube to stay up to date on the latest news, events and innovations that will help you face and solve your toughest challenges.
Do you want to reuse or translate content?
Just post a link to the entry and send us a quick note so we can share your work. Thank you very much.
Our Global Community
Emerson Exchange 365
The opinions expressed here are the personal opinions of the authors. Content published here is not read or approved by Emerson before it is posted and does not necessarily represent the views and opinions of Emerson.