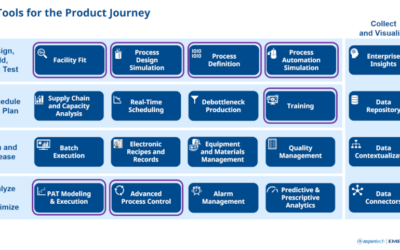
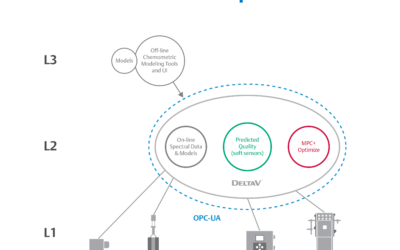
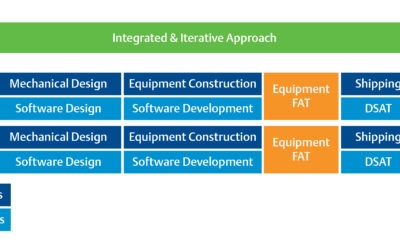
There’s no question that today’s life sciences landscape is changing dramatically. As new personalized medicine solutions emerge, companies are rapidly changing scale to include more multi-product manufacturing facilities. Many of these facilities are manufacturing...
Posts by or with Michalle Adkins
Data Integrity in Life Sciences Podcast
In this Emerson Automation Experts podcast, Michalle Adkins and Hilary Mills-Baker join me to discuss the challenges of achieving the required data integrity and solutions to drive performance improvements.
Achieving Data Integrity, Quality & Compliance in Manufacturing
In a recent webinar, Achieve Data Integrity, Quality & Compliance Across Your Organization, Emerson’s Michalle Adkins and Hilary Mills-Baker share how data integrity means complete, consistent, accurate data throughout its lifecycle.
Life Sciences Digital Maturity – Know Where You Are to Know Where You Can Go
To know where you’re going, you have to know where you are, and operating a life sciences manufacturing facility is no exception. Pharmaceutical processors regularly have to take stock of their technological capacity to keep a finger on the pulse of the four main...
Digital Solutions for Process Development, Tech Transfer and Manufacturing
Emerson’s Michalle Adkins and AspenTech’s Chuck Miller team up to present Robust PAT Solutions to Support Process Development, Tech Transfer and Manufacturing at IFPAC 2023.
Traceability Across the Entire Drug Development Pipeline
Recently, as cell and gene therapies have begun to emerge in the life sciences manufacturing landscape, an important concept has begun to draw attention: traceability. However, it is important to remember that traceability was critical long before today’s most...
Four Pillars of Life Sciences Speed to Market
People are more aware than ever of the many amazing ways new treatments can improve their lives. As a result, life sciences companies are under more pressure than ever to safely deliver new, innovative treatments to market to satisfy customer demand. The key to...
Improving Digital Maturity is Key to Life Sciences Success
In just the last 10 years, biopharmaceutical supply chains have changed dramatically. The single-source manufacturing model is becoming much rarer, making way for multi-enterprise manufacturing collaborations and partnerships to better meet the changing needs of the...
Module Type Package Unlocks the Flexibility Driving Pharma Innovation
There can be little question that Industry 4.0 technologies are reshaping the way life sciences companies operate. In the wake of the rapid development of vaccines during the COVID-19 pandemic, more facilities than ever are being “born digital.” Some of these...
Let your Personnel Lead you to the Adaptive Plant
On Monday at the Emerson Exchange Users’ Conference, Emerson’s Michalle Adkins and AbbVie’s Jeff Ziolkowski moderated a panel with Fujifilm’s Soren Skerris, Takeda’s Dr. Nina Schwalb, Eli Lilly’s James Weber, and Emerson’s Christian Berg. The experts shared the...
BioPhorum’s Roadmap for Speed to Market with In-Line Monitoring and Real-Time Release
Traditional manufacturing practices have served the biopharmaceutical industry well for years, but times are changing and a need for speed to market in life sciences while reducing manufacturing costs is putting a focus on enabling real-time release (RTR)....
Shorten the Equipment Validation Process to Bring Projects Online Faster
One of the key stages delaying and increasing costs in life sciences projects is equipment validation. In fact, it is one of the elements of project completion that project teams are most eager to compress because the value of every incremental improvement is so...
Follow Us
We invite you to follow us on Facebook, LinkedIn, Twitter and YouTube to stay up to date on the latest news, events and innovations that will help you face and solve your toughest challenges.
Do you want to reuse or translate content?
Just post a link to the entry and send us a quick note so we can share your work. Thank you very much.
Our Global Community
Emerson Exchange 365
The opinions expressed here are the personal opinions of the authors. Content published here is not read or approved by Emerson before it is posted and does not necessarily represent the views and opinions of Emerson.