Last week, IFPAC 2023 was held in Maryland. If you’re unfamiliar with IFPAC, here is a description from their website.
IFPAC is the essential meeting place for the latest developments in Process Analytical Technology…PAT, Quality by Design…QbD, and overall process monitoring & control within the Pharmaceutical, Biotechnology, Food, Chemical, Petrochemical & related industries.
Emerson’s Michalle Adkins and AspenTech’s Chuck Miller teamed up to present Robust PAT Solutions to Support Process Development, Tech Transfer and Manufacturing. Michalle opened the presentation by describing the challenges pharmaceutical and biopharmaceutical manufacturers face in bringing products to market. These challenges include accelerating the research & development pipeline, making manufacturing processes more flexible to accommodate rapid product changeover, meeting environmental, social & governance (ESG) corporate goals, accelerating the quality & product release processes, and having consistency and repeatability in meeting production schedules.
To address these challenges, Michalle highlighted some digital solutions across many parts of the product journey. These parts include design, build & test, plan & schedule, run & release, analyze & optimize, and collect & visualize. Here is a look at the digital solutions that can help address each part’s challenges.
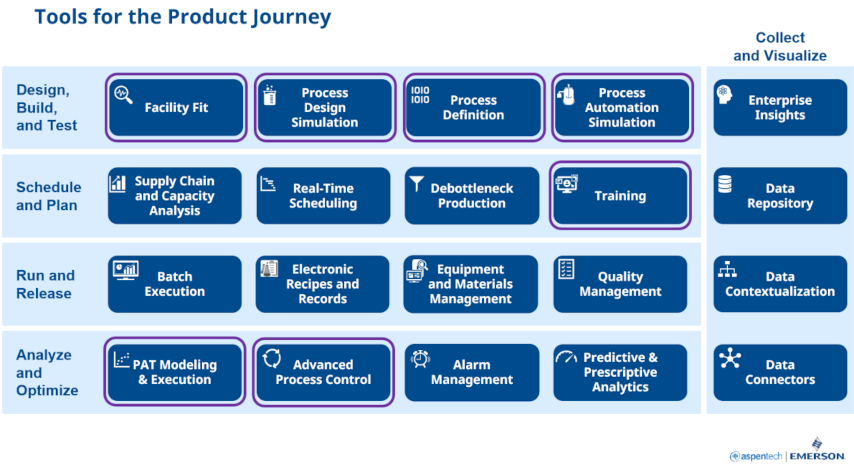
Process and knowledge management solutions, such as Emerson PKM, help to define the product and process to bridge process science to manufacturing, facilitate collaboration across the product lifecycle, streamline the tech transfer process, reduce time to market, and transfer recipe parameters to plant execution and automation systems across the enterprise. This solution helps you manage risks across the product lifecycle and evaluate facility fit.
Process design simulations, such as AspenPlus, Aspen Batch Process Developer, and Aspen Properties, aid in developing a process from early process route selection to full-scale manufacturing. These simulations include data-driven, hybrid, and first principles models for physical properties, pure components, mixtures, and estimation & regression. Recipe-driven batch process models help evaluate alternatives, develop cost estimates, generate material balances, select synthetic routes for new molecules, perform cycle-time calculations, and calculate air emissions. Simulations using Mimic software, can extend to the automation system control logic, dynamic process and I/O simulation, and immersive virtual reality.
Chuck followed this up by drilling down into the solutions that address several key workflows in PAT. In the R&D phase of the product journey, where process understanding and optimization are important, the time-tested exploratory multivariate tools in Aspen Unscrambler and Aspen ProMV are particularly useful. When it comes to model build and validation for on-line PAT models, the wide range of modeling methods and flexible fit for purpose model validation of Aspen Unscrambler enable efficient workflows for GxP model management.
For on-line PAT method deployment, the complementary offerings of Aspen Process Pulse and DeltaV Spectral PAT enable flexibility and scalability to accommodate widely varying usage scenarios. Aspen Process Pulse supports around 30 analyzers and data sources, is highly scalable, and supports BYO and 3rd party models and instruments. DeltaV Spectral PAT is particularly effective for running the model and closing the loop within the control system with its ready-to-go advanced process controls with simplified & robust model execution and streamlined validation.
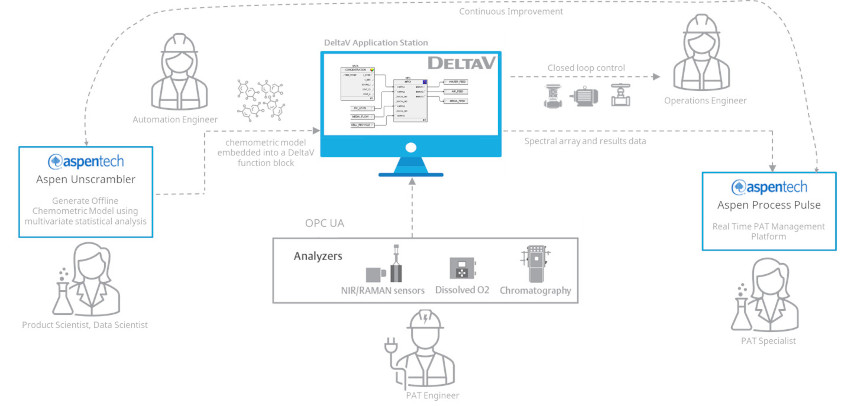
Finally, once a method is deployed at commercial scale, our on-line PAT platforms are designed to support other critical workflows, including results visualizations, deviation detection and diagnosis, retrospective investigations, continued process verification (CPV) reporting, model updating, and method change management.
Follow the links above for more information on these essential digital solutions to drive improved product lifecycle performance.

