Pharmaceutical and biotech manufacturers seek to improve both project and operational performance through implementation of smart factory technology. The smart factory fits right into the S95 enterprise automation model and provides out-of-box manufacturing...
ISA-88
Challenge of Designing PCS-Driven MES Architectures for a Greenfield Facility
Author: Jonathan Lustri I have previously written about a design strategy where the process control system (PCS) is the primary system driving all procedural batch activity within a pharmaceutical process. In this architecture, the PCS ISA-88 procedural model must...
Optimizing Batch Process Automation Design in MES and DCS
For pharmaceutical and biotech manufacturers, the flow and capture of information is as important as the flow of the manufacturing process in readying products to release for sale. In a Pharmaceutical Technology magazine article, Connecting MES to Process Control,...
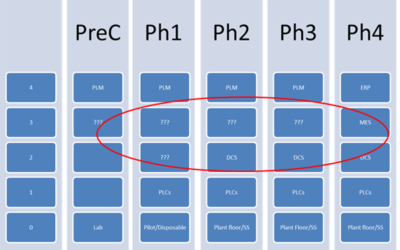
Improving Technology Transfer by Earlier Adoption of Standards and Software Platforms
In 2011, the U.S. Food & Drug Administration (FDA) published their Guidance for Industry – Process Validation: General Principles and Practices. A PharmManufacturing.com article, A Framework for Technology Transfer to Satisfy the Requirements of the New Process...
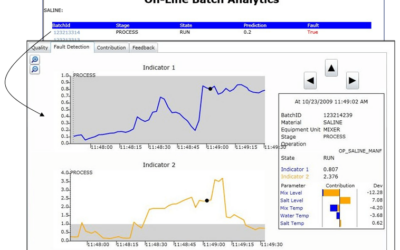
Applying Batch Analytics to Fermentation Processes
Creating batch process models such as fermentation is challenging due to their inherent time variability batch to batch. It's also critical to know when problems are beginning to develop when corrective measures can be taken. Emerson's David Rehbein, a Senior Data...
Mistake Proofing Pharmaceutical Production and Documentation Processes
As a follow up to the post, Using Operational Excellence Techniques to Understand and Solve Business Problems, Emerson's Michalle Adkins shared a great story of one pharmaceutical manufacturer's path to justify technology to help their business objective to reduce...
Welcome Mark Nixon to the Modeling and Control Blog
I wanted to welcome a new blogger to the family of Emerson Process Management bloggers. Emerson's Mark Nixon joins Terry Blevins and Greg McMillan over at the Modeling and Control blog. You may recall Mark from some earlier posts on this blog. I've known Mark for many...
Optimizing Sequence-Based Control Strategies
Update and bump: The whitepaper, DCS Controller Loading Reduction for Sequence Logic - A Boiler Control Project Case Study, is now available. Original post: Let's close this week with a post about sequence control loading improvements in boiler operations. I managed...
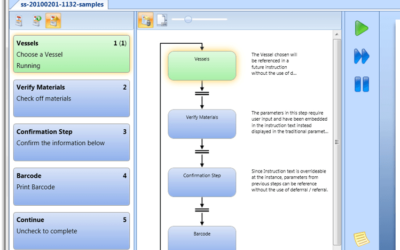
Paperless, Release by Exception Electronic Workflows
If your current work processes are causing delays in getting your manufactured products released for sale, you may want to catch Christie Deitz', Electronic Workflow for a Bioreactor presentation. She'll be co-presenting with an automation engineer from a leading...
Applying the ISA88 Model to Clean In Place Operations
Every industry has its special jargon that is like a secret handshake. If you're an insider, you can quickly spot the outsiders based upon their understanding of your industry's jargon and acronyms. For instance, my background was in the offshore oil and gas business...
Keep Up to Date With the Latest News and Updates
Follow Us
We invite you to follow us on Facebook, LinkedIn, Twitter and YouTube to stay up to date on the latest news, events and innovations that will help you face and solve your toughest challenges.
Do you want to reuse or translate content?
Just post a link to the entry and send us a quick note so we can share your work. Thank you very much.
Our Global Community
Emerson Exchange 365
The opinions expressed here are the personal opinions of the authors. Content published here is not read or approved by Emerson before it is posted and does not necessarily represent the views and opinions of Emerson.