Author: Jonathan Lustri I have previously been interviewed and written about a process control and MES [Manufacturing Execution Systems] architecture where the batch control logic within the process control system is the single procedural engine. It drives procedural...
operations management system
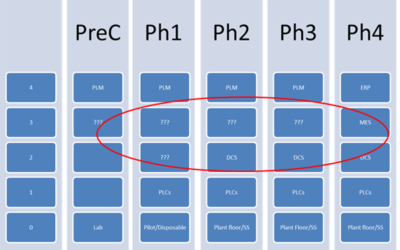
Challenge of Designing PCS-Driven MES Architectures for a Greenfield Facility
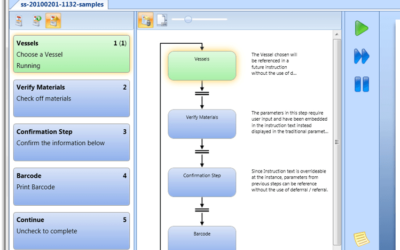
Author: Jonathan Lustri I have previously written about a design strategy where the process control system (PCS) is the primary system driving all procedural batch activity within a pharmaceutical process. In this architecture, the PCS ISA-88 procedural model must...
Deploying Syncade Manufacturing Execution System in a Non-English Language
Author: Jonathan Lustri I recently worked on a Syncade manufacturing execution system (MES) project where the system was deployed in Danish. Emerson executes all its projects with a global team and this one was no different. Our customer was Danish and we had team...
Niche-Buster Medicine Information Solutions
Author: Michalle Adkins We are seeing a trend in the life sciences industry moving away from blockbusters to niche busters, population based medicines and even to the extreme of personalized medicines. The Emerson Life Sciences consulting team recently had the...
Data Integrity for Compliance, Audit Readiness and Performance
Pharmaceutical and biotech manufacturers have stringent regulatory compliance requirements. These requirements must balance with the need for improvement and optimization to run safely, efficiently, profitably and in line with the business and quality objectives. In a...
Improving Technology Transfer by Earlier Adoption of Standards and Software Platforms
In 2011, the U.S. Food & Drug Administration (FDA) published their Guidance for Industry – Process Validation: General Principles and Practices. A PharmManufacturing.com article, A Framework for Technology Transfer to Satisfy the Requirements of the New Process...
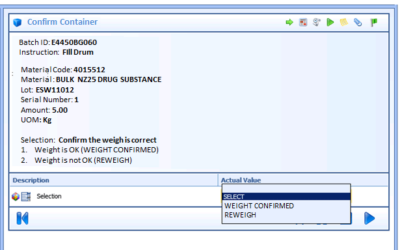
Electronically Dispatching Work Procedures
I saw a tweet yesterday, which pointed to a great BioProcess International article, Biopharmaceutical Information Infrastructure 2.0 (Part 1 of 2). I sent the link to Emerson's Jonathan Lustri, who manages the Syncade smart operations management brand for his...
Keep Up to Date With the Latest News and Updates
Follow Us
We invite you to follow us on Facebook, LinkedIn, Twitter and YouTube to stay up to date on the latest news, events and innovations that will help you face and solve your toughest challenges.
Do you want to reuse or translate content?
Just post a link to the entry and send us a quick note so we can share your work. Thank you very much.
Our Global Community
Emerson Exchange 365
The opinions expressed here are the personal opinions of the authors. Content published here is not read or approved by Emerson before it is posted and does not necessarily represent the views and opinions of Emerson.