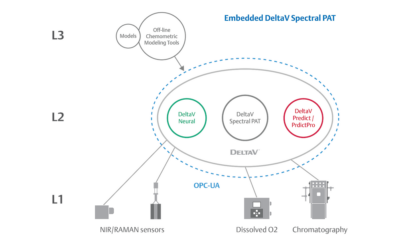
One of Pharma Manufacturing magazine’s Pharma Innovation Award winners is DeltaV Spectral Process Analytic Technology (PAT) which helps pharma and biopharma manufacturers build a foundation for better regulatory management while optimizing facility performance and throughput.
critical quality attributes
Successfully Implementing Process Analytical Technology
It's been well more than a decade since the U.S. Food & Drug Administration (FDA) announced a Process Analytical Technology (PAT) approach for pharmaceutical manufacturers in their Guidance for Industry PAT — A Framework for Innovative Pharmaceutical Development,...
Is Process Analytical Technology Rocket Science?
More than a decade ago, the U.S. Food and Drug Administration published, Guidance for Industry PAT — A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance. In this document, the FDA explained: The scientific, risk-based framework...
Clarifying Design Space, Process Analytical Technology and Quality by Design
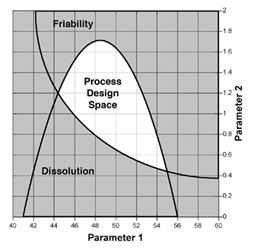
For pharmaceutical and biopharmaceutical manufacturers, there are a lot confusion around the concepts of design space (DS), Process Analytical Technology (PAT), and Quality by Design (QbD). Emerson's Zuwei Jin believes that this confusion has largely limited the...
Continued Process Verification in the Process Validation Lifecycle
In 2011, the U.S. Food and Drug Administration (FDA) issued a Guidance for Industry – Process Validation: General Principles and Practices. It highlighted a third validation stage goal of continued process verification (CPV) for: …continual assurance that the process...
Enabling Release by Exception Manufacturing
The Emerson Exchange conference October 6-10 in Orlando, Florida USA is rapidly approaching. I caught up with Emerson's Michalle Adkins who mentioned that her team of Life Sciences consultants would be highlighting the enablement of release by exception. Although...
Implementing Process Analytical Technology and Continuous Process Verification
In the 4:42 video, Life Science Drug Process Development and Manufacturing, Emerson's Gary Mitchell highlights the changes occurring for pharmaceutical and biotech manufacturers. Gary opens noting how these manufacturers are challenged to respond to new methods for...
Implementing Quality by Design
Many manufacturers in the Life Sciences industry are challenged to respond to a new paradigm for process development and manufacturing. The FDA's cGMP for the 21st Century initiative is driving the industry to change its development and manufacturing to be based on...
Continuous Manufacturing for Solid Dose Form Pharmaceuticals
Emerson's Jonathan Lustri explores the trend towards continuous manufacturing for solid dose pharmaceuticals and the technologies and practices that make this possible. This past year I have become aware of a very big change that is coming to the pharmaceutical...
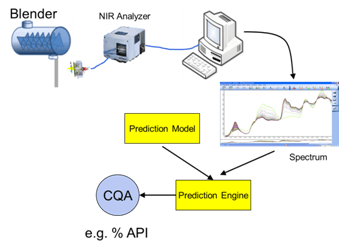
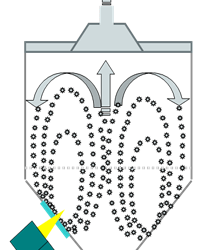
Fluidized Bed and Bin Blender PAT Applications
In the early 2000s, a Wall Street Journal article, New Prescription For Drug Makers: Update the Plants, shared the news: The FDA [U.S. Food and Drug Administration] has concluded that the industry needs to adopt manufacturing innovations, partly to raise quality...
Keep Up to Date With the Latest News and Updates
Follow Us
We invite you to follow us on Facebook, LinkedIn, Twitter and YouTube to stay up to date on the latest news, events and innovations that will help you face and solve your toughest challenges.
Do you want to reuse or translate content?
Just post a link to the entry and send us a quick note so we can share your work. Thank you very much.
Our Global Community
Emerson Exchange 365
The opinions expressed here are the personal opinions of the authors. Content published here is not read or approved by Emerson before it is posted and does not necessarily represent the views and opinions of Emerson.