Within the operations and safety stream of the Emerson Exchange EMEA 2024 conference in Düsseldorf, Astrid Aguilar Fajardo and Matthijs Niemeijer from Getinge described how Emerson’s DeltaV™ automation system is helping to redefine the journey bioprocessing companies are taking to bring new drugs to the market.

The presentation won the award for most innovative application for operations and safety excellence at the Emerson Exchange EMEA 2024.
Getinge is a global medical technology company providing equipment and systems within healthcare and life sciences. The Getinge Applikon division supplies bioreactor systems offering full automation, cleanability and traceability for use within research and development (R&D) through to full-scale production.
Aguilar Fajardo explained that the introduction of new drugs to the market can often take over 20 years when including the initial drug discovery period, pre-clinical development, clinical trials, FDA review and large-scale manufacturing. Therapies are becoming ever more complex, with increased process complexity and more time spent on pre-clinical development. When transitioning from one phase to the next, production/process optimization requires translation of recipes (tech transfer) between the laboratory and commercial applications. Simplifying tech transfer correlates directly into accelerating time-to-market and thus is a key area of focus for bioprocessing companies.
Digitalization is an essential element. The presentation outlined the five levels within the digital plant maturity model and stated that (according to BioProcess International magazine) 77% of bioprocessing companies have developed, or are currently developing, digitalization strategies. Such strategies will usually include automation (platforms to facilitate modeling for process automation), data storage and standardization (data management, storage and standardization of metadata, cloud systems and data exchange formats), data analysis and modeling (in-house/off-the-shelf packages, data quality in modeling, digital twins, predictive decision-support tools) and regulatory concerns (legal considerations for modeling in healthcare, ethical and responsible application).
Getinge is helping its customers in the bioprocessing industry to meet some of the challenges they face, especially those related to tech transfer. Aguilar Fajardo explained that scaling up between different process development phases from discovery to production can be challenging. Data and technology transfer within a multi-software platform environment can be very time-consuming, while translation from one platform to another also increases the risks of errors.
The presentation described the Applikon V-Control software platform from Getinge, which has been designed to manage bioreactors used within drug discovery to full production. The platform, developed through Emerson’s OEM program for life sciences, combines an Applikon bioreactor system from Getinge with Emerson’s DeltaV automation system to provide process control, automation and data acquisition functions.
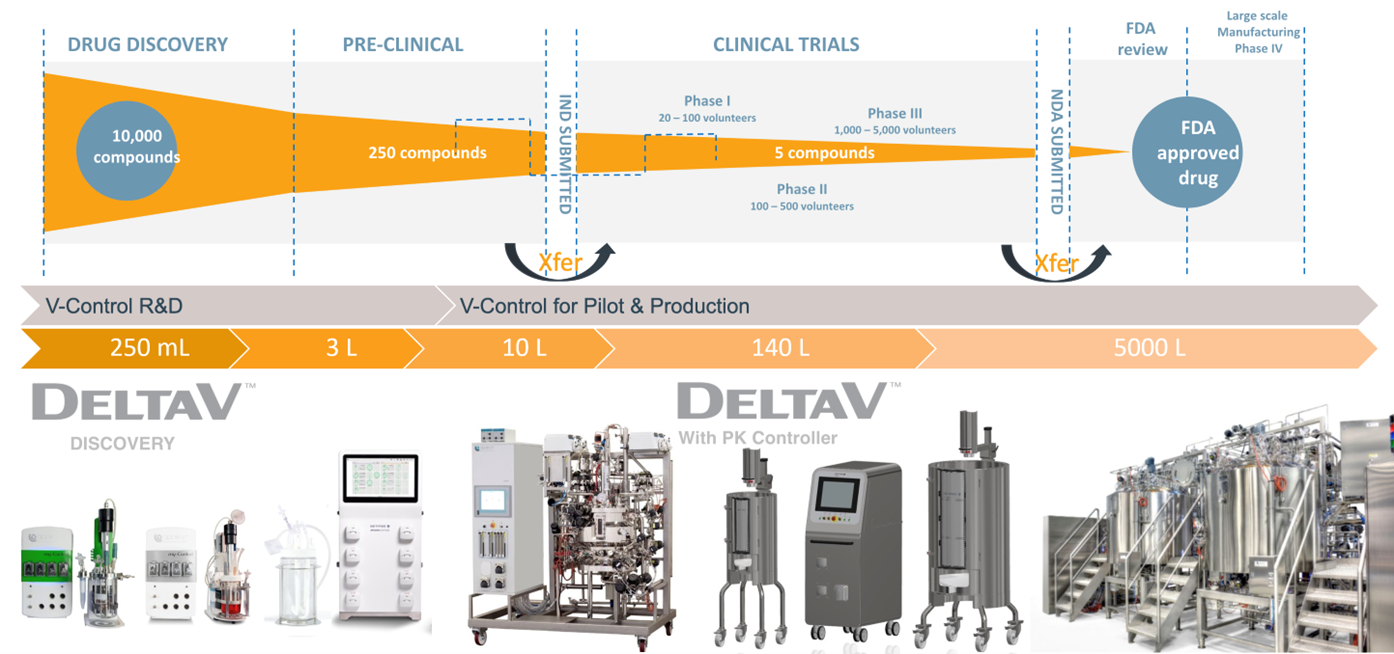
DeltaV enables a single platform to be used, from R&D to production, reducing the time to bring new drugs to the market.
A key component of the solution is DeltaV Discovery, which has been specifically designed to support bioreactors used in drug discovery and pre-clinal development, non-production applications. As the customer transitions from drug discovery to pilot and full production (multi or single-use systems), the DeltaV PK Controller is applied in a configurable control system based on the user’s requirements. Critically, the solution enables a single automation platform to be used from R&D through to full production. That allows seamless technology transfer and scalable data transfer, resulting in optimal bioprocesses with shorter development lead times and lower development costs.
A range of additional software tools from Emerson and AspenTech were highlighted that support pipeline acceleration, manufacturing time savings, reduced downtime and faster product release. An example was provided of the V-Control bioreactor system with DeltaV deployed at a Getinge customer operating in the USA and India. The solution also included integrated Spectral Process Analytic Technology (PAT) to enable continuous online monitoring and control of critical process parameters (CPPs) that affect critical quality attributes (CQAs) of the product. DeltaV PredictPro function blocks for modeling and model predictive control are being used to ensure consistency in product quality and quantity, while Aspen Process Pulse software from AspenTech is deployed to provide real-time verification and data visualization, reporting and audit trail functions, ensuring GMP compliancy.
In summary, Aguilar Fajardo revealed that by using a common automation platform in R&D and production, users can achieve savings of up to 40% in re-work and validation costs, less expenditure on training requirements for new operators, and improved flexibility in R&D.