Initiating Good Manufacturing Practices (GMP) production operations, whether as part of facility start-up, product changeover, or following a shutdown, requires complex coordination of both logical and physical process controls. Inefficient configuration and qualification practices for automation and information management systems can lead to both start-up delays and productivity losses. A few items among the many to orchestrate for production readiness are
- Logical changeover
- Physical changeover
- Data integration
Logical changeover incurs time spent ensuring facility fit, configuring systems, testing for intended use, and validating the final automation and information management solutions. In many cases, this body of work slows the introduction of new products and can impede changeover between products.
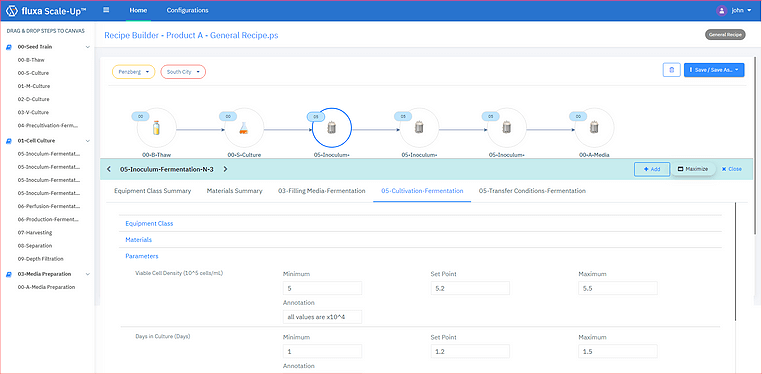
One strategy to streamline logical changeover activities is to focus on implementing flexible product recipe configurations by leveraging parameterization. Recipe design is a key element of manufacturing operations management, and consistent speed of configuration deployment can be facilitated by targeted parameterization of product-specific information. Fluxa PKM helps to streamline the product parameterization process, helping deployment teams focus on using standardized re-usable recipe object to simplify deployment and validation efforts.
Another key element to Production Readiness is physical changeover. Maintenance planning, schedule misalignment, improper equipment status, delayed equipment cleaning or sterilization, and long commissioning times can cause significant delays and unnecessary operating costs.
Application of single-use systems is a rapidly emerging approach to streamline physical changeover. One challenge with leveraging single-use systems is ensuring proper component status and set-up. As an example, pH instruments need to be verified for proper performance as part of set-up; however, that verification often can’t happen until after product has been introduced into the system. When considering single-use systems, it is important to ensure that all components of that system can be verified for status and performance prior to introduction of product. Next generation pH probes offer this capability and are purpose built for the challenges introduced with single-use systems.
Data is an often-overlooked co-product of cGMP processes. A well-conceived data integration strategy helps further facilitate effective Production Readiness activities. Complex system landscapes, numerous custom interfaces, a wide varieties of data types, and islands of automation result in data integrity and cybersecurity problems. The building blocks for process data originate in the logical and physical systems that make up the manufacturing landscape. Emerson offers a number of solutions to facilitate data integration and to deliver the regulatory and continuous improvement value of process information.
Emerson’s Life Sciences team is here to provide automation expertise and technology to help regulated manufacturers increase operating efficiency and optimize production. Check out our products and service offerings to see how we can help you achieve your vision of operational success.