When we hear the phrase “personalized healthcare,” many of us think about the medicines and therapeutics focused on our specific needs that deliver treatments to dramatically improve or restore our health and extend our lives. In a Pharma Focus Asia webinar, Pursuing the Adaptive Plant to Enable Personalized Healthcare, Emerson’s Christian Berg provides a broader perspective of personalized healthcare and its challenges for the Life Sciences industry.
Christian opened his presentation by noting that Cell Therapy and Gene Therapy treatments have emerged in this past decade as viable treatments for life-threatening diseases. These treatments are referred to as Advanced Therapeutic Medicinal Products (ATMP). For manufacturers in the Life Sciences industry, ATMP manufacturing has highlighted growing challenges, including:
- Increased supply chain complexity
- Smaller production volumes
- Higher frequency of batches
- Higher frequency of new product introduction, tech transfer, and product changeover.
Two types of cell therapies are Allogeneic Cell Therapy (donor is not the patient) and Autologous Cell Therapy (donor is the patient). Gene Therapy is a modification of the expression of an individual’s genes with the administration of a specific nucleic acid (DNA or RNA). These nucleic acids require special carriers called vectors. These vectors can be viral or non-viral.
He then broadened the scope of personalized healthcare. The evolution is away from traditional healthcare, which includes these characteristics:
- Diagnostics are regulated as a function of statistical repeatability and reproducibility studies.
- Treatment protocols are a function of population-based studies, dependent upon study participant selection using population-based diagnostics.
- Variations within clinical study populations limit treatment efficacies.
- Manufacturers are incentivized to produce large batches based on population-derived demand forecasts.
- Therapeutics are centrally produced and distributed globally.
Personalized healthcare includes these characteristics.
- Precision, patient-specific diagnostics regulated as a function of controlled processes.
- Treatment protocols are a function of precision diagnostic generated bioinformatics leading to patient-specific courses of treatment.
- Treatment efficacy is not limited to confidence intervals constrained by variations within the population; treatment that leads to cure is an expectation for all patients.
- Manufacturers are incentivized to minimize batch sizes in favor of real-time production.
- Manufacturing supply chains are flexible, with localized production capabilities to deliver specific therapeutics as close to real-time as possible.
Christian demonstrates how personalized healthcare works. Like traditional healthcare, it begins with the patient discovering the need for medical attention. The healthcare provider isolates the condition using traditional diagnostics such as blood samples, x-rays, CAT scans, MRIs, etc. Based on the results of the diagnostics, the patients are grouped based on expressed disease characteristics. In traditional healthcare, large-batch prescription therapies are applied to address the condition.
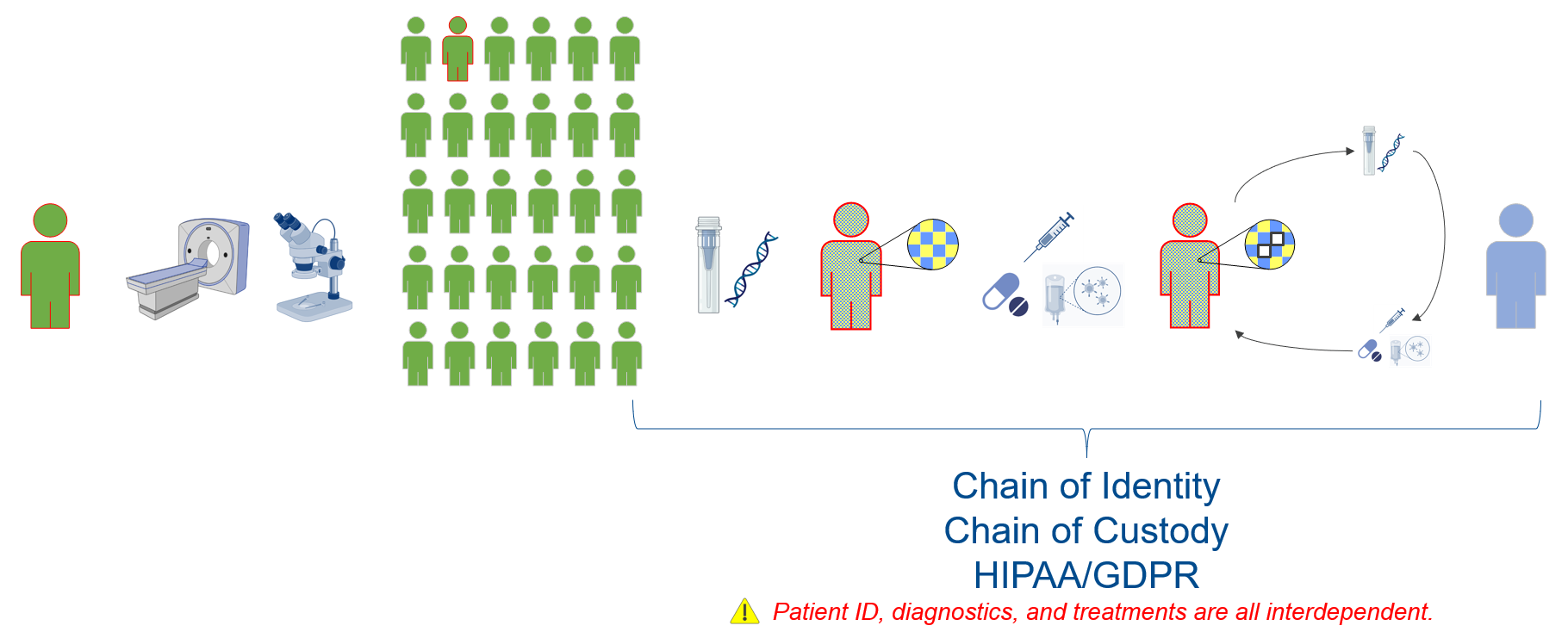
Where personalized healthcare separates from traditional healthcare is precision molecular diagnostics to create molecular bioinformatics used in developing individualized treatment plans. Treatment efficacy and progress are monitored with minimal residual disease (MRD) diagnostics. The individualized treatment (no longer population-based) is modified as appropriate based on these MRD diagnostics. The continuous improvement cycle of treatment, monitoring, and medication continues until the disease is no longer detected.
For biopharmaceutical manufacturers, this new approach introduces a host of challenges. Treatment is not solely a function of therapeutics; it is a function of patient-specific treatment response. Tracking must be performed from manufacturing through the individualized treatment lifecycle. Data connectivity and integrity are critical and must transcend the manufactured batch. This data connectivity for traceability and control extends to the chains of identity and custody. It is compliant with regulations such as HIPAA and GDPR for patient ID, diagnostic results, and treatments.
He identifies the challenges biopharmaceutical manufacturers face in this transition to personalized healthcare.
- Small scale, high-frequency manufacturing leads to more opportunities for defects
- High volume of production records leads to records retention challenges
- High volume of production records leads to quality investigation challenges
- Patient-specific components increase the risk profile for product mix-ups
Christian highlighted the work of the BioPhorum Operations Group and their Digital Plant Maturity Model, highlighting increasing levels of maturity—pre-digital plant, digital silos, connected plant, predictive plant, and adaptive plant. Addressing the challenges requires advancement to the highest level with an adaptive plant. Characteristics of an adaptive plant include:
- Full end-to-end value-chain integration from suppliers to patients.
- Modular, mobile, and collaborative Manufacturing Environment.
- Advanced production technologies used as standard.
- “Plug-n-play everything” from an instrument to a production scale or a CMO.
- Zero system downtime (including upgrades) – continuous evolution.
- In-line, real-time, continuous, closed loop, process verification and control with automated real-time quality release.
- Self-aware, continuously adaptive, “Autonomous” plant; exception conditions handled by remote experts.
- Advanced simulation across the value chain for modeling, testing, and improving manufacturing and supporting business processes.
- Trusted information insights are freely and securely available.
- Pervasive use of adaptive analytics and Self / Machine learning across the value chain.
In part 2 of this post, we’ll explore the technology approaches which must adapt, the changes required in traditional system integrations, and the technologies and solutions needed to address these challenges with an adaptive plant.
Visit the Life Sciences & Medical section on Emerson.com for more on the technologies and solutions to help drive your personalized healthcare supplier strategies.

