In a U.S. Food and Drug Administration's FDA Voice blog post, FDA Budget Matters: Investing in Advanced Domestic Manufacturing, FDA Commissioner Dr. Scott Gottlieb opened noting: Advanced manufacturing, which includes various technologies, such as continuous...
biologics
Optimizing Media Flow in Biologics Manufacturing
Author: Emily Anderson Flexible facilities are a growing trend in the biopharmaceutical manufacturing industry. Manufacturers increasingly look to produce multiple products and maximize production time. To meet these requirements, an automation strategy must maximize...
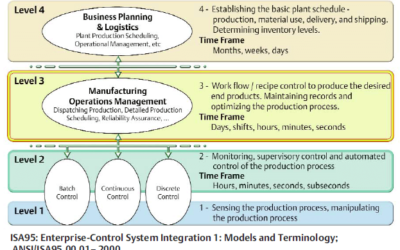
Bridging Islands of Automation
While integration technologies have helped bridge "islands of automation" over the years, some process manufacturers still have parts of their automated processes disconnected from the rest. Emerson's Gary Mitchell, a senior industry consultant on the Life Sciences...
Keep Up to Date With the Latest News and Updates
Follow Us
We invite you to follow us on Facebook, LinkedIn, Twitter and YouTube to stay up to date on the latest news, events and innovations that will help you face and solve your toughest challenges.
Do you want to reuse or translate content?
Just post a link to the entry and send us a quick note so we can share your work. Thank you very much.
Our Global Community
Emerson Exchange 365
The opinions expressed here are the personal opinions of the authors. Content published here is not read or approved by Emerson before it is posted and does not necessarily represent the views and opinions of Emerson.