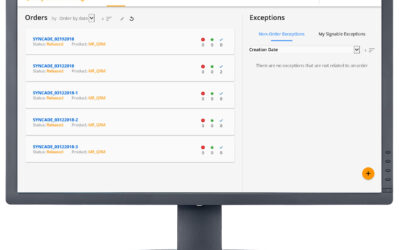
Exceptions in batch manufacturing processes are the deviations that occur outside the prescribed specifications. For pharmaceutical and biopharmaceutical manufacturers, quality and manufacturing personnel must review these exceptions. Traditionally, this quality...
Improving Batch Manufacturing Quality Review Cycle Time
read more