A biological drug is defined as a:
…substance that is made from a living organism or its products and is used in the prevention, diagnosis, or treatment of cancer and other diseases. Biological drugs include antibodies, interleukins, and vaccines.
The market for biological drugs continues to rapidly grow. In fact, in China alone, there were 201 Biological New Drug Applications (NDAs) in 2016, a 32% increase over 2015.
I caught up with Emerson’s Zuwei Jin who will be visiting Shanghai and Beijing to speak with an audience of more than 200 pharmaceutical and biopharmaceutical manufacturers about automation in the pharmaceutical industry. The Chinese pharmaceutical industry is challenged to meet the Current Good Manufacturing Practices (cGMPs) regulations established by the U.S. Food & Drug Administration (FDA). These regulations:…provide for systems that assure proper design, monitoring, and control of manufacturing processes and facilities.
Quality standards and validation is a hot topic among Chinese manufacturers and there is an active effort to better understand the Process Validation standards—Quality by Design (QbD) and Risk Assessment.
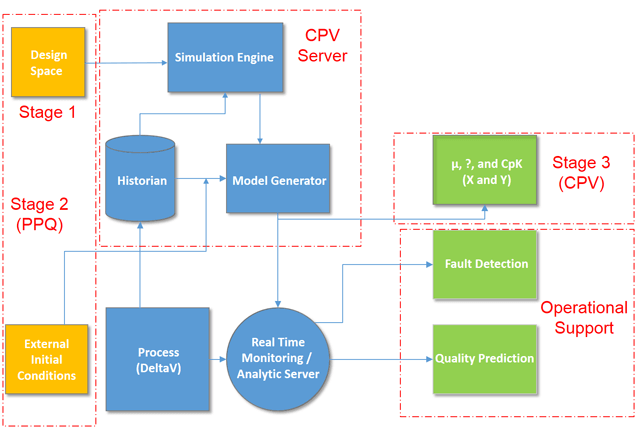
Zuwei will share how Big Data and process analytics provide the basis for continued process verification (CPV). CPV is the third stage of process validation after stage 1—process design and stage 2—process qualification. Incorporating CPV into the automation strategy helps to provide ongoing assurance that the process remains within its designed operating ranges.
Techniques such as Multi-variate Data Analysis (MVDA) have historically been used on longstanding commercial batch processes with a long record of historical batches. Online MVDA is now practical in stage 2 using a patented method of a CPV server that includes a historian, a simulation running Monte Carlo methods, a design space quality predictor and batch analyzer.
This approach allows the Life Sciences manufacturer to start with their vision for the design space and develop an MVDA model from one historical batch. Simulated batches are used in this model building process. Instead of using CPV just in stage 3, data management can be integrated across all three stages of the drug development cycle.
Integrated Data Management helps reduce financial and business risks across the lifecycle in areas such as technology transfer, automation standardization, enterprise-level recipe management and incorporation of new technologies such as the Industrial Internet of Things and Industrie 4.0.
You can connect and interact with other pharmaceutical and biotech manufacturing experts in the Life Sciences group of the Emerson Exchange 365 community.