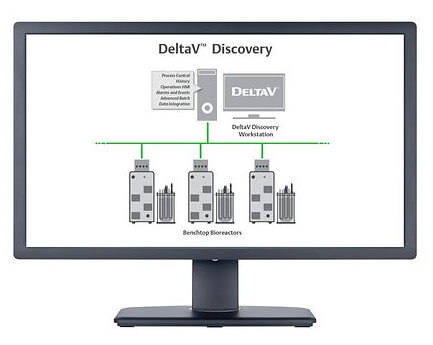
 Emerson Exchange begins in just a few short days. I am quite excited about the Life Sciences relevant content in the exhibit hall this year. We are covering many different technologies including instrumentation, controls, batch processing, process analytical technology integration, manufacturing execution systems (MES), analytics, virtual reality, augmented reality, IIOT, and more. We will have several bioreactors: R&D sized with DeltaV Discovery, single use with instruments using Syncade MES for set up instructions, and small stainless-steel bioreactor with the DeltaV PK controller.
Emerson Exchange begins in just a few short days. I am quite excited about the Life Sciences relevant content in the exhibit hall this year. We are covering many different technologies including instrumentation, controls, batch processing, process analytical technology integration, manufacturing execution systems (MES), analytics, virtual reality, augmented reality, IIOT, and more. We will have several bioreactors: R&D sized with DeltaV Discovery, single use with instruments using Syncade MES for set up instructions, and small stainless-steel bioreactor with the DeltaV PK controller.
I am thrilled to see some of our vision for Life Sciences really starting to come to fruition. Drivers in the industry include speed to market, operational excellence, and cost of compliance. The solutions shown at this event support these drivers. If you are fortunate to attend, some of the solution themes you will see revolve around faster technology transfer, plug and play, single use technologies, quality review, continuous manufacturing, digital twin, and more.
I will be at the single use station in the Exhibits hall where we will have single use sensors and devices including: pH, dissolved oxygen, pressure, and pinch valves. I’ll also be stepping through an electronic workflow as an example of how you could use this capability for set up of single use manufacturing operations. I hope to see you there.




