Sean Buckley and co-authors from Roche, Takeda, and BioPhorum author CGT [cell & gene therapy] Actors and Process Maps which highlight six common types of CGT showing how the pattern of actors and process blocks varies by therapy type.
biopharmaceuticals
BioPhorum Cell and Gene Therapy Personas and User Stories
The BioPhorum CGT Personas and User Stories toolkit details the needs of all the key players involved in end-to-end cell and gene therapy (CGT) processes. It can be used by anyone who wishes to better understand how IT systems can support the manufacture and delivery of CGTs.
Optimizing Biopharmaceutical Manufacturing Processes
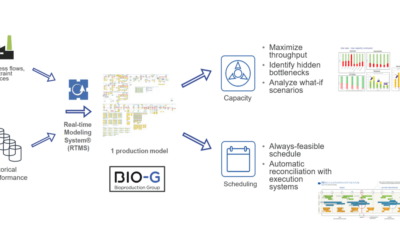
Emerson’s Alan Johnston shares how technology advances in real-time modeling, adaptive scheduling and predictive maintenance are enabling successes in optimization for biopharmaceutical manufacturers who may have a combination of both types of processes in their operations.
BioPhorum Collaboration for Improving Biopharmaceutical Manufacturing
Emerson’s Michalle Adkins, Benjamin Arriola & Brandon Haschke are co-authors along with professionals in leading biopharmaceutical manufacturing and suppliers in the BioPhorum work, In-line monitoring / real-time release testing in biopharmaceutical processes – prioritization and cost benefit analysis.
Oral Solid Drug Continuous Manufacturing
In a May 19 BioPharma Asia webinar, Continuous Manufacturing as a Default Platform for Oral Solid Drug Products, Emerson’s Bob Lenich will team up with Johnson and Johnson Senior Principal Engineer Lawrence De Belder to discuss the path to continuous manufacturing for these types of medicines.
BioPhorum Best Practices on Knowledge Management
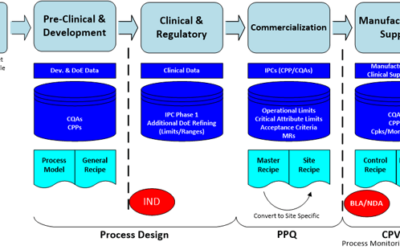
Emerson’s Michalle Adkins alerted me to the release of a new BioPhorum Knowledge Management white paper, A Test Case in CMC Business Processes from Late-Stage Development to Commercial Manufacturing.
Accelerating the Development and Production of Therapeutics
The focus of the world right now is on how to defeat the Coronavirus and return to more normal times. Manufacturers in the Life Sciences industry are racing at a breakneck pace to develop vaccines and treatments. Automation, analytics play a large role in both the...
Biopharmaceutical Models in Development and Production
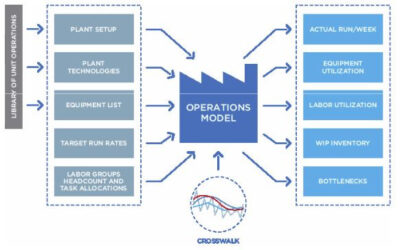
Models of manufacturing and production processes can provide value through the lifecycle of these facilities, from up front design through ongoing personnel training and optimization. In a BioPharm International article, Biopharma modeling, now and five years from no…
Biomanufacturing Fluidic System Single-Use Technologies
As medicines and therapies become more specialized for individuals, the requirements of biopharmaceutical manufacturing processes are dramatically changing, especially in the transition from large-scale to single-use production processes. In a Processing magazine...
Advancing Digital Plant Maturity for Biopharmaceutical Manufacturers
At the recent 2019 China International Biopharma4.0 Summit, Emerson's Ron Rossbach presented Biopharma 4.0—Integrated Software Enabling Digital Capabilities. I'll highlight some of Ron's key points. He opened describing some of the main challenges biopharmaceutical...
Biopharmaceutical Single Use Measurement and Control
At this past autumn’s Emerson Exchange conference in San Antonio, Texas, Emerson’s Michalle Adkins demonstrated single use manufacturing measurement and control devices and how they could be used to measure and control biomanufacturing operations in a single use environment.
Advancing the Management of Data through Life Sciences Product Lifecycles
From a recent Life Sciences symposium, we looked at issues in the advancement of process intelligence and analytics. Today we'll look at another work session from the symposium, Managing Data Through the Product Life Cycle. The general session was led by Amgen's...
Keep Up to Date With the Latest News and Updates
Follow Us
We invite you to follow us on Facebook, LinkedIn, Twitter and YouTube to stay up to date on the latest news, events and innovations that will help you face and solve your toughest challenges.
Do you want to reuse or translate content?
Just post a link to the entry and send us a quick note so we can share your work. Thank you very much.
Our Global Community
Emerson Exchange 365
The opinions expressed here are the personal opinions of the authors. Content published here is not read or approved by Emerson before it is posted and does not necessarily represent the views and opinions of Emerson.