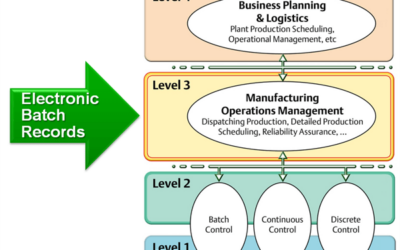
The days of maintaining paper records across the life sciences development and production pipeline are gone. Today’s regulatory requirements, not to mention the complexity of new treatments, requires organization that can only be provided electronically. However,...
EBR
Making Medicine Personal with Flexible Automation Solutions
Personalized medicine manufacturers are rapidly changing their manufacturing platforms to provide a new scale of production. In the Emerson Exchange Virtual Series, life sciences experts held an open Q&A on the trajectory of cell and gene therapies, and how a...
Electronic Batch Record Design Considerations
Last week, the 2nd International Summit on GMP, GCP & Quality Control was held in Chicago, Illinois USA. Emerson's Heather Schwalje, a senior Life Sciences consultant, presented Moving beyond part 11; Quality assurance considerations for translating Current Good...
Paperless, Release by Exception Electronic Workflows
If your current work processes are causing delays in getting your manufactured products released for sale, you may want to catch Christie Deitz', Electronic Workflow for a Bioreactor presentation. She'll be co-presenting with an automation engineer from a leading...
Keep Up to Date With the Latest News and Updates
Follow Us
We invite you to follow us on Facebook, LinkedIn, Twitter and YouTube to stay up to date on the latest news, events and innovations that will help you face and solve your toughest challenges.
Do you want to reuse or translate content?
Just post a link to the entry and send us a quick note so we can share your work. Thank you very much.
Our Global Community
Emerson Exchange 365
The opinions expressed here are the personal opinions of the authors. Content published here is not read or approved by Emerson before it is posted and does not necessarily represent the views and opinions of Emerson.