The BioPhorum Operations Group (BPOG) established a digital plant maturity model (DPMM) for biopharmaceutical manufacturing to define stages of factory evolution “from simple paper-based plants through to the fully automated and integrated ‘adaptive plant’ of the future.” The model gives organizations a common tool for industry comparison and measurement of progress. It also provides a platform to facilitate collaboration within the life sciences industry and its technology providers.
Fluxa PKM
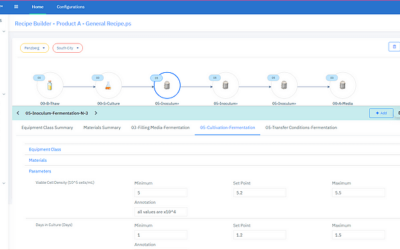
Holistic Production Readiness Strategy is Key to Operational Success
Initiating Good Manufacturing Practices (GMP) production operations, whether as part of facility start-up, product changeover, or following a shutdown, requires complex coordination of both logical and physical process controls. Inefficient configuration and qualification practices for automation and information management systems can lead to both start-up delays and productivity losses.
Improving Logical Changeover Production Readiness in the Life Sciences
Technology has continued to advance and provide ways to assist pharmaceutical & biopharmaceutical manufacturers with production readiness. Improper set up and qualification of automation and information management production applications cause start-up and product changeover delays, as well as slow performance during production runs.
Keep Up to Date With the Latest News and Updates
Follow Us
We invite you to follow us on Facebook, LinkedIn, Twitter and YouTube to stay up to date on the latest news, events and innovations that will help you face and solve your toughest challenges.
Do you want to reuse or translate content?
Just post a link to the entry and send us a quick note so we can share your work. Thank you very much.
Our Global Community
Emerson Exchange 365
The opinions expressed here are the personal opinions of the authors. Content published here is not read or approved by Emerson before it is posted and does not necessarily represent the views and opinions of Emerson.