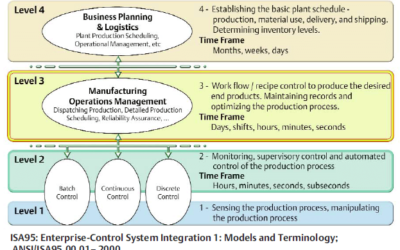
Pharmaceutical and biotech manufacturers seek to improve both project and operational performance through implementation of smart factory technology. The smart factory fits right into the S95 enterprise automation model and provides out-of-box manufacturing...
S95
Data Integrity for Compliance, Audit Readiness and Performance
Pharmaceutical and biotech manufacturers have stringent regulatory compliance requirements. These requirements must balance with the need for improvement and optimization to run safely, efficiently, profitably and in line with the business and quality objectives. In a...
Successfully Automating Terminal Operations
Making an operational step change in a production process involves a clear vision and people, processes and tools to execute the vision. A July/August 2014 Tank Storage magazine article, Automating terminal operations, highlights the story of Vopak transforming their...
Enabling Release by Exception Manufacturing
The Emerson Exchange conference October 6-10 in Orlando, Florida USA is rapidly approaching. I caught up with Emerson's Michalle Adkins who mentioned that her team of Life Sciences consultants would be highlighting the enablement of release by exception. Although...
Improving Technology Transfer by Earlier Adoption of Standards and Software Platforms
In 2011, the U.S. Food & Drug Administration (FDA) published their Guidance for Industry – Process Validation: General Principles and Practices. A PharmManufacturing.com article, A Framework for Technology Transfer to Satisfy the Requirements of the New Process...
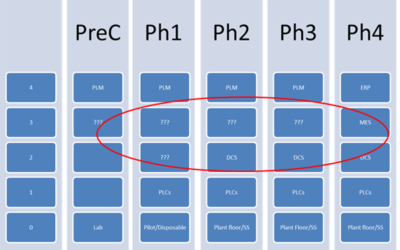
Bridging Islands of Automation
While integration technologies have helped bridge "islands of automation" over the years, some process manufacturers still have parts of their automated processes disconnected from the rest. Emerson's Gary Mitchell, a senior industry consultant on the Life Sciences...
Decisions for Operations Management Architectures
At the request of one of Emerson Process Experts blog readers who has long commute to and from his office, I started to add podcast recordings at the end of each post to provide an audio version of each post, beginning this past October. Today, I thought we'd push the...
Keep Up to Date With the Latest News and Updates
Follow Us
We invite you to follow us on Facebook, LinkedIn, Twitter and YouTube to stay up to date on the latest news, events and innovations that will help you face and solve your toughest challenges.
Do you want to reuse or translate content?
Just post a link to the entry and send us a quick note so we can share your work. Thank you very much.
Our Global Community
Emerson Exchange 365
The opinions expressed here are the personal opinions of the authors. Content published here is not read or approved by Emerson before it is posted and does not necessarily represent the views and opinions of Emerson.