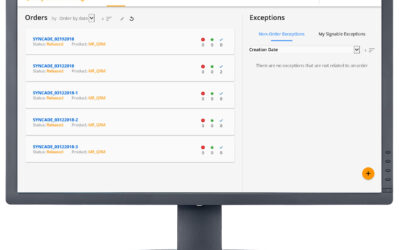
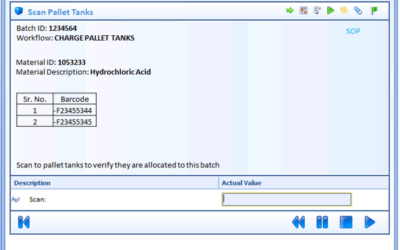
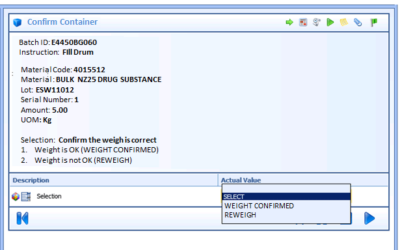
Exceptions in batch manufacturing processes are the deviations that occur outside the prescribed specifications. For pharmaceutical and biopharmaceutical manufacturers, quality and manufacturing personnel must review these exceptions. Traditionally, this quality...
Syncade
Identify and Resolve Cell Therapy Batch Manufacturing Exceptions
We conclude our series on improving cell therapy manufacturing with a closer look at the review by exception process. In the manufacturing process of cell therapeutics, product cycle times are extremely short and any exceptions occurring during the production process...
Designing and Building Pharmaceutical and Biotech Smart Factories
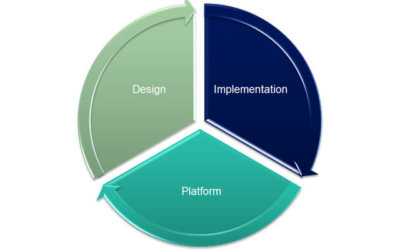
Pharmaceutical and biotech manufacturers seek to improve both project and operational performance through implementation of smart factory technology. The smart factory fits right into the S95 enterprise automation model and provides out-of-box manufacturing...
Digital Transformation to Paperless Production
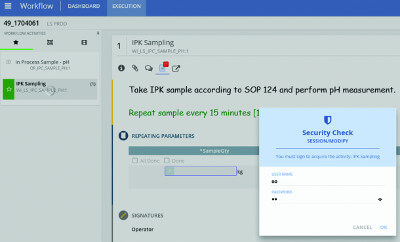
Manufacturing processes have long been paper-based with log entries, initialing, signoffs and other transactions to manually record. While automation systems have captured some of this manual recordkeeping, they have not been able to capture everything. In batch...
Reducing Execution Errors in Cell Therapeutics Manufacturing
In an earlier post, Managing the Chain of Identity in Cell Therapeutics Manufacturing, we highlighted how the manufacturing process of these cell therapies must be performed with absolute accuracy the first time since each batch is produced for a specific patient....
Managing the Chain of Identity in Cell Therapeutics Manufacturing
Medicines produced by pharmaceutical and biotech manufacturers are manufactured in many ways. One particular type of treatment, cell therapy, is defined as: …the transplantation of human or animal cells to replace or repair damaged tissue. From a manufacturing...
Improving Batch Manufacturing Workflows and Recordkeeping
The pharmaceutical and biotechnology manufacturing industries are challenged with errors in manual entries in the production process, getting batches right the first time, reaching final signoff for batch release, and managing the data accumulated throughout the...
Improving Raw Material Management Productivity
For manufacturers in highly-regulated industries, such as pharmaceutical and biopharmaceutical manufacturing, it is critical to keep track of raw materials as part of the production records. To minimize or avoid waste, it's also important to use the first-to-expire...
Challenge of Designing PCS-Driven MES Architectures for a Greenfield Facility
Author: Jonathan Lustri I have previously written about a design strategy where the process control system (PCS) is the primary system driving all procedural batch activity within a pharmaceutical process. In this architecture, the PCS ISA-88 procedural model must...
Best Practices Specifying MES Workflows for Life Sciences Projects
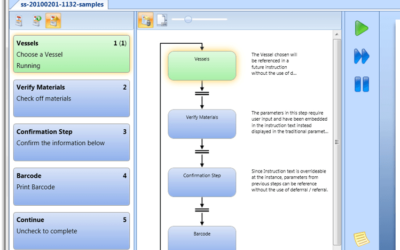
Author: Jonathan Lustri Manufacturing Execution System (MES) Life Sciences projects can be costly and be a challenge to keep in control. The biggest contributor to these challenges are the defining and design of manufacturing workflows. A Workflow is an MES procedural...
Integrated Biometric Authentication in Electronic Records
How many of you have more passwords than you can possibly remember? Count me in with the affirmatives. Biometric authentication is one way to overcome this large and growing problem. For process manufacturers and producers, especially those in highly regulated...
Deploying Syncade Manufacturing Execution System in a Non-English Language
Author: Jonathan Lustri I recently worked on a Syncade manufacturing execution system (MES) project where the system was deployed in Danish. Emerson executes all its projects with a global team and this one was no different. Our customer was Danish and we had team...
Keep Up to Date With the Latest News and Updates
Follow Us
We invite you to follow us on Facebook, LinkedIn, Twitter and YouTube to stay up to date on the latest news, events and innovations that will help you face and solve your toughest challenges.
Do you want to reuse or translate content?
Just post a link to the entry and send us a quick note so we can share your work. Thank you very much.
Our Global Community
Emerson Exchange 365
The opinions expressed here are the personal opinions of the authors. Content published here is not read or approved by Emerson before it is posted and does not necessarily represent the views and opinions of Emerson.