Yesterday we highlighted advancements in exception management technology for pharmaceutical and biopharmaceutical manufacturers. Exception management is one element in an electronic batch record (EBR). Other elements for the EBR which also have high data integrity...
21 CFR Part 11
Integrated Biometric Authentication in Electronic Records
How many of you have more passwords than you can possibly remember? Count me in with the affirmatives. Biometric authentication is one way to overcome this large and growing problem. For process manufacturers and producers, especially those in highly regulated...
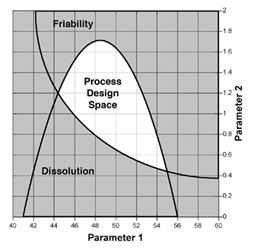
Implementing Quality by Design
Many manufacturers in the Life Sciences industry are challenged to respond to a new paradigm for process development and manufacturing. The FDA's cGMP for the 21st Century initiative is driving the industry to change its development and manufacturing to be based on...
Continuous Process Verification per FDA Process Validation Guidance
It was just about a year ago that the U.S. Food and Drug Administration published their Guidance for Industry - Process Validation: General Principles and Practices. I caught up with Emerson's Heather Schwalje, a senior consultant on the Life Sciences industry team....
Planning an Electronic Document Management Project
It's great that Tim Alosi, from Emerson local business partner, New England Controls, was in town this past week. He saw the recent post, Shrinking Batch Record Creation Time and Document Management Complexity. Repligen is one of New England Controls customers, and...
Keep Up to Date With the Latest News and Updates
Follow Us
We invite you to follow us on Facebook, LinkedIn, Twitter and YouTube to stay up to date on the latest news, events and innovations that will help you face and solve your toughest challenges.
Do you want to reuse or translate content?
Just post a link to the entry and send us a quick note so we can share your work. Thank you very much.
Our Global Community
Emerson Exchange 365
The opinions expressed here are the personal opinions of the authors. Content published here is not read or approved by Emerson before it is posted and does not necessarily represent the views and opinions of Emerson.