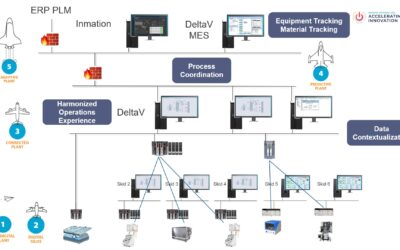
Emerson’s Bruce Greenwald, Christian Berg, and David Gray collaborated to present “Solutions for Advanced Therapeutics” at the Emerson Exchange 2025 Conference.
biotech manufacturing
Simplifying Recipe Creation in the Life Sciences Podcast
Melissa Lee & Kebra Tynan join Jim Cahill in this podcast to discuss how Emerson is helping Life Sciences manufacturers address drug development lifecycle challenges with a software-as-a-service (SaaS) based workflow management solution.
Enabling Boundless Automation in the Life Sciences Industry
Several of our recent Life Sciences podcasts have featured conversations about the rapid shifts taking place in this dynamic industry. Automation and information management technology are rapidly advancing to help address these challenges. In a recent Emerson...
Trends, Challenges and Solutions in the Life Sciences Industry Podcast
In this Emerson Automation Experts podcast, Dave Imming joins Jim Cahill to discuss some of Life Sciences industry trends, the challenges they have introduced, and the role technology can play in addressing them.
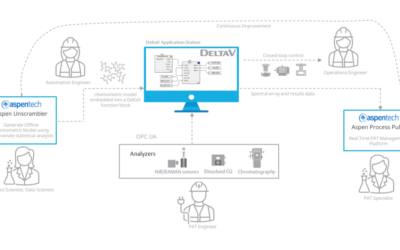
DeltaV Spectral PAT for Life Sciences Podcast
In this podcast, Emerson’s Jorge Costa and Bruce Greenwald join Jim Cahill to discuss the application of PAT technologies not only within the Life Sciences but across many of the process and hybrid manufacturing industries.
Data Integrity in Life Sciences Podcast
In this Emerson Automation Experts podcast, Michalle Adkins and Hilary Mills-Baker join me to discuss the challenges of achieving the required data integrity and solutions to drive performance improvements.
Achieving Data Integrity, Quality & Compliance in Manufacturing
In a recent webinar, Achieve Data Integrity, Quality & Compliance Across Your Organization, Emerson’s Michalle Adkins and Hilary Mills-Baker share how data integrity means complete, consistent, accurate data throughout its lifecycle.
Driving Operational Improvements with a Rugged PAT Model Infrastructure
At the IFPAC-2023 meeting, Emerson’s Bruce Greenwald and Aspen Technology’s Geir Rune Flåten teamed together to present, Deploying a Rugged PAT Model Infrastructure for Real-Time Monitoring and Control.
Prototype Sensing Technologies are at the Forefront of Innovation
On June 4-7, experts in process analytical technology (PAT) gathered for the 2023 IFPAC conference. While there, they discussed the latest developments in PAT, quality by design, and overall process monitoring and control within the pharmaceutical, biotechnology,...
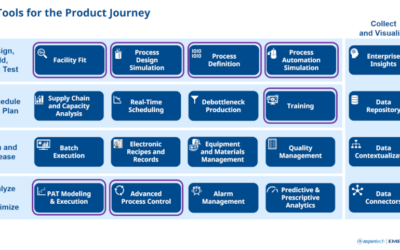
Digital Solutions for Process Development, Tech Transfer and Manufacturing
Emerson’s Michalle Adkins and AspenTech’s Chuck Miller team up to present Robust PAT Solutions to Support Process Development, Tech Transfer and Manufacturing at IFPAC 2023.
Better Treatment—For the Earth, and its People
It can be hard to keep up with the many rapid changes occurring in the life sciences industry these days. In an effort to embrace improved speed to market, sustainability, and quality, life sciences manufacturers are employing a wide range of software and technologies...
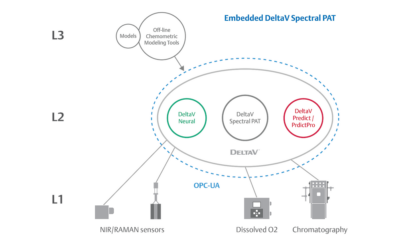
Embedded Spectral Process Analytic Technology
One of Pharma Manufacturing magazine’s Pharma Innovation Award winners is DeltaV Spectral Process Analytic Technology (PAT) which helps pharma and biopharma manufacturers build a foundation for better regulatory management while optimizing facility performance and throughput.
Keep Up to Date With the Latest News and Updates
Follow Us
We invite you to follow us on Facebook, LinkedIn, Twitter and YouTube to stay up to date on the latest news, events and innovations that will help you face and solve your toughest challenges.
Do you want to reuse or translate content?
Just post a link to the entry and send us a quick note so we can share your work. Thank you very much.
Our Global Community
Emerson Exchange 365
The opinions expressed here are the personal opinions of the authors. Content published here is not read or approved by Emerson before it is posted and does not necessarily represent the views and opinions of Emerson.