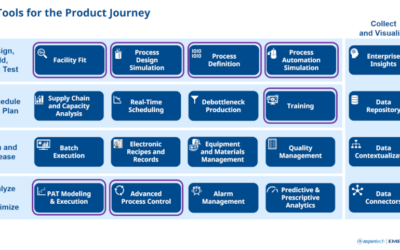
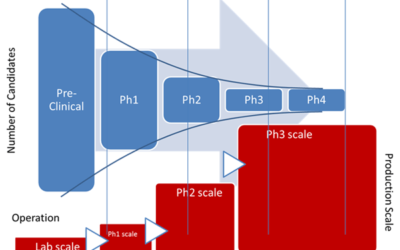
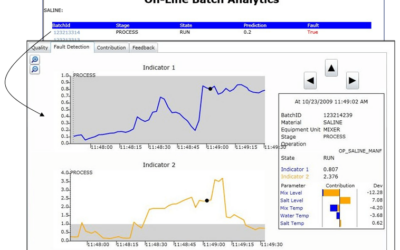
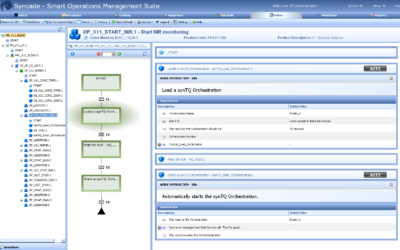
On June 4-7, experts in process analytical technology (PAT) gathered for the 2023 IFPAC conference. While there, they discussed the latest developments in PAT, quality by design, and overall process monitoring and control within the pharmaceutical, biotechnology,...
IFPAC
Digital Solutions for Process Development, Tech Transfer and Manufacturing
Emerson’s Michalle Adkins and AspenTech’s Chuck Miller team up to present Robust PAT Solutions to Support Process Development, Tech Transfer and Manufacturing at IFPAC 2023.
Biopharmaceutical Product and Process Knowledge Management
For biopharmaceutical producers, creating value from the knowledge capture and dissemination is paramount. At the upcoming February 23-26 IFPAC [International Foundation Process Analytical Chemistry] conference, Emerson’s Michalle Adkins will present on the impact of KM in product and process development for biopharmaceutical manufacturers.
Online Multivariate Data Analysis for Continued Process Verification
Measuring quality in real-time improves the overall performance of a manufacturing process. The International Foundation Process Analytical Chemistry (IFPAC) is: …a world-wide, not-for-profit organization dedicated to the advancement of Process Analytical Technology...
Applying Batch Analytics to Fermentation Processes
Creating batch process models such as fermentation is challenging due to their inherent time variability batch to batch. It's also critical to know when problems are beginning to develop when corrective measures can be taken. Emerson's David Rehbein, a Senior Data...
Faster Product Releases with PAT Methods in Production Recipes
This week, the International Foundation Process Analytical Chemistry (IFPAC) is holding their 26th international forum and exhibition in Baltimore, Maryland USA. At this event, Emerson's Chris Amstutz, Life Sciences industry consulting team director, gave a...
Keep Up to Date With the Latest News and Updates
Follow Us
We invite you to follow us on Facebook, LinkedIn, Twitter and YouTube to stay up to date on the latest news, events and innovations that will help you face and solve your toughest challenges.
Do you want to reuse or translate content?
Just post a link to the entry and send us a quick note so we can share your work. Thank you very much.
Our Global Community
Emerson Exchange 365
The opinions expressed here are the personal opinions of the authors. Content published here is not read or approved by Emerson before it is posted and does not necessarily represent the views and opinions of Emerson.