The life sciences marketplace is changing faster than nearly any other industry. Gone are the days when companies focus on a single treatment to drive their entire business. Today’s life sciences manufacturers are challenged to bring new treatments to the marketplace...
manufacturing execution system
BioPhorum MES of the Future
In BioPhorum’s MES of the Future Manifesto, Sean and Kate Porter collaborated with other biomanufacturing leaders on requirements for future Manufacturing Execution Systems.
Digital Transformation Key to Vaccine Development and Rollout
Accelerating vaccine development requires unprecedented flexibility in managing data. With increasing integration between product lifecycle management systems and software—like distributed control systems (DCS) and manufacturing execution systems (MES)—data, recipes, and processes can be digitally altered, shared, and used to scale across the lifecycle.
Manufacturing Execution Systems in Drug Research and Development
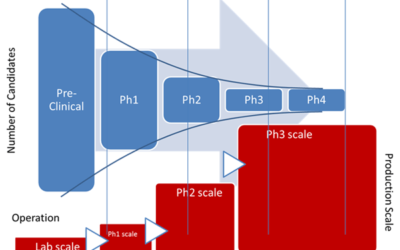
The U.S. Food & Drug Administration (FDA) outlines the drug development process in five steps: Discovery and development Preclinical research Clinical trials FDA review FDA post-market safety monitoring I caught up with Emerson's Zuwei Jin whom you may recall from...
Tracking Cell Therapy Chain of Identity
In this 2:25 YouTube video, Address Cell Therapy Batch Production Challenges with Syncade, Emerson’s Michalle Adkins shares how operations management technology plays an important role in addressing these challenges.
Best Practices for Implementing a Process Control Driven MES System
Author: Jonathan Lustri I have previously been interviewed and written about a process control and MES [Manufacturing Execution Systems] architecture where the batch control logic within the process control system is the single procedural engine. It drives procedural...
Moving to Electronic Batch Records
Yesterday we highlighted advancements in exception management technology for pharmaceutical and biopharmaceutical manufacturers. Exception management is one element in an electronic batch record (EBR). Other elements for the EBR which also have high data integrity...
Improving Batch Manufacturing Quality Review Cycle Time
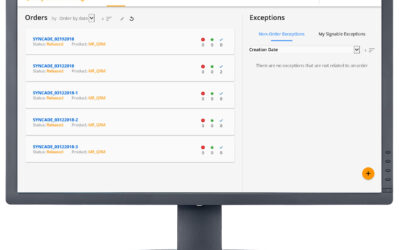
Exceptions in batch manufacturing processes are the deviations that occur outside the prescribed specifications. For pharmaceutical and biopharmaceutical manufacturers, quality and manufacturing personnel must review these exceptions. Traditionally, this quality...
Tech Transfer in Drug Development Pipeline and Industry 4.0
Author: Zuwei Jin Among all of the important trends in life sciences industry such as modular plants, integrated modules, out-of-box smart factory, single-use equipment, PAT, and manufacturing execution systems (MES), IIOT has been receiving high interest including...
Identify and Resolve Cell Therapy Batch Manufacturing Exceptions
We conclude our series on improving cell therapy manufacturing with a closer look at the review by exception process. In the manufacturing process of cell therapeutics, product cycle times are extremely short and any exceptions occurring during the production process...
Digital Transformation to Paperless Production
Manufacturing processes have long been paper-based with log entries, initialing, signoffs and other transactions to manually record. While automation systems have captured some of this manual recordkeeping, they have not been able to capture everything. In batch...
Reducing Execution Errors in Cell Therapeutics Manufacturing
In an earlier post, Managing the Chain of Identity in Cell Therapeutics Manufacturing, we highlighted how the manufacturing process of these cell therapies must be performed with absolute accuracy the first time since each batch is produced for a specific patient....
Keep Up to Date With the Latest News and Updates
Follow Us
We invite you to follow us on Facebook, LinkedIn, Twitter and YouTube to stay up to date on the latest news, events and innovations that will help you face and solve your toughest challenges.
Do you want to reuse or translate content?
Just post a link to the entry and send us a quick note so we can share your work. Thank you very much.
Our Global Community
Emerson Exchange 365
The opinions expressed here are the personal opinions of the authors. Content published here is not read or approved by Emerson before it is posted and does not necessarily represent the views and opinions of Emerson.