In this Emerson Automation Experts podcast, Michalle Adkins and Hilary Mills-Baker join me to discuss the challenges of achieving the required data integrity and solutions to drive performance improvements.
Michalle Adkins
Achieving Data Integrity, Quality & Compliance in Manufacturing
In a recent webinar, Achieve Data Integrity, Quality & Compliance Across Your Organization, Emerson’s Michalle Adkins and Hilary Mills-Baker share how data integrity means complete, consistent, accurate data throughout its lifecycle.
Increasing Life Sciences Manufacturing Capacity
In an Australasian BioTechnology article, Facilities for a Strong Future, Emerson’s Michalle Adkins and Makarand Mujumdar share how pharmaceutical and biotech manufacturers can improve local capacity to meet the needs of the regions in which they operate.
Improving Logical Changeover Production Readiness in the Life Sciences
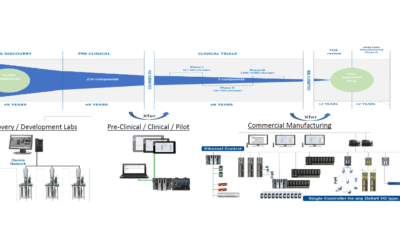
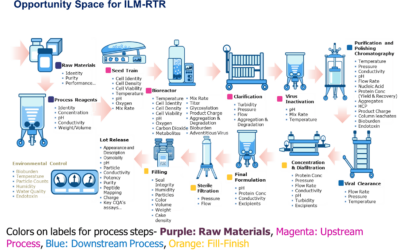
Technology has continued to advance and provide ways to assist pharmaceutical & biopharmaceutical manufacturers with production readiness. Improper set up and qualification of automation and information management production applications cause start-up and product changeover delays, as well as slow performance during production runs.
Building Cross-Cultural Awareness
At a recent Emerson Exchange Americas virtual presentation session, Emerson’s Michalle Adkins and Alpana Desai presented on communicating with cross-cultural awareness.
Improving Product and Process Development in Life Sciences
Emerson’s Michalle Adkins will be discussing villains and heroes in knowledge management at the fully digital November 2-6, 2020 ISPE Annual Meeting and Expo.
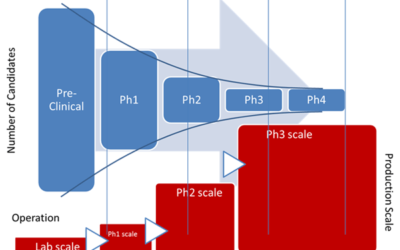
Accelerate Technology Transfer through the Product Development Pipeline
Emerson’s Michalle Adkins and Ron Rossbach will host a webinar on reducing time to market for new therapies—a key opportunity for pharmaceutical and biopharmaceutical manufacturers. This webinar will take place on August 13 at 15:00 CET – Europe / 09:00 EDT.
BioPhorum Collaboration for Improving Biopharmaceutical Manufacturing
Emerson’s Michalle Adkins, Benjamin Arriola & Brandon Haschke are co-authors along with professionals in leading biopharmaceutical manufacturing and suppliers in the BioPhorum work, In-line monitoring / real-time release testing in biopharmaceutical processes – prioritization and cost benefit analysis.
BioPhorum Best Practices on Knowledge Management
Emerson’s Michalle Adkins alerted me to the release of a new BioPhorum Knowledge Management white paper, A Test Case in CMC Business Processes from Late-Stage Development to Commercial Manufacturing.
Biopharmaceutical Product and Process Knowledge Management
For biopharmaceutical producers, creating value from the knowledge capture and dissemination is paramount. At the upcoming February 23-26 IFPAC [International Foundation Process Analytical Chemistry] conference, Emerson’s Michalle Adkins will present on the impact of KM in product and process development for biopharmaceutical manufacturers.
Innovating to Meet Bioprocessing Single-Use Requirements
Suppliers to the industry have been innovating to meet the single-use requirements of biotech manufacturers. Emerson’s Michalle Adkins pointed me to a recent award received for technology innovation for these single-use requirements.
BioPhorum Roadmap for In-line Monitoring and Real-time Release
Emerson’s Michalle Adkins will be presenting, Update on BioPhorum’s Roadmap for In-line Monitoring and Real- Time Release at the conference.
Keep Up to Date With the Latest News and Updates
Follow Us
We invite you to follow us on Facebook, LinkedIn, Twitter and YouTube to stay up to date on the latest news, events and innovations that will help you face and solve your toughest challenges.
Do you want to reuse or translate content?
Just post a link to the entry and send us a quick note so we can share your work. Thank you very much.
Our Global Community
Emerson Exchange 365
The opinions expressed here are the personal opinions of the authors. Content published here is not read or approved by Emerson before it is posted and does not necessarily represent the views and opinions of Emerson.