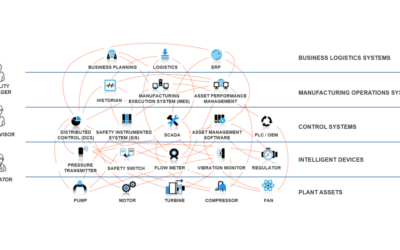
In this podcast, Emerson’s Jorge Costa and Bruce Greenwald join Jim Cahill to discuss the application of PAT technologies not only within the Life Sciences but across many of the process and hybrid manufacturing industries.
pharmaceutical manufacturing
Data Integrity in Life Sciences Podcast
In this Emerson Automation Experts podcast, Michalle Adkins and Hilary Mills-Baker join me to discuss the challenges of achieving the required data integrity and solutions to drive performance improvements.
Achieving Data Integrity, Quality & Compliance in Manufacturing
In a recent webinar, Achieve Data Integrity, Quality & Compliance Across Your Organization, Emerson’s Michalle Adkins and Hilary Mills-Baker share how data integrity means complete, consistent, accurate data throughout its lifecycle.
Driving Operational Improvements with a Rugged PAT Model Infrastructure
At the IFPAC-2023 meeting, Emerson’s Bruce Greenwald and Aspen Technology’s Geir Rune Flåten teamed together to present, Deploying a Rugged PAT Model Infrastructure for Real-Time Monitoring and Control.
Digital Solutions for Process Development, Tech Transfer and Manufacturing
Emerson’s Michalle Adkins and AspenTech’s Chuck Miller team up to present Robust PAT Solutions to Support Process Development, Tech Transfer and Manufacturing at IFPAC 2023.
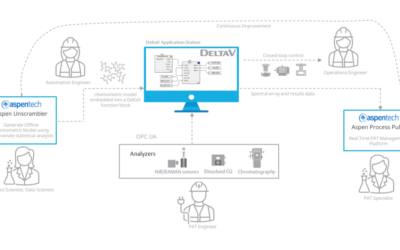
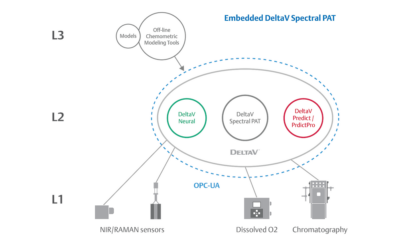
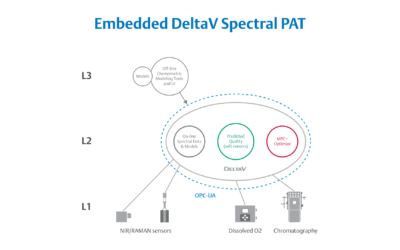
Embedded Spectral Process Analytic Technology
One of Pharma Manufacturing magazine’s Pharma Innovation Award winners is DeltaV Spectral Process Analytic Technology (PAT) which helps pharma and biopharma manufacturers build a foundation for better regulatory management while optimizing facility performance and throughput.
Real-World Implications of Spectral PAT for Life Sciences
Emerson offers an integrated Spectral PAT solution for DeltaV, allowing manufacturers to pursue closed-loop control (and real-time quality assessments) using in-line spectroscopic instruments.
Manufacturing Solutions in Personalized Healthcare
Christian Berg describes some solutions and technologies which facilitate the pursuit of the adaptive plant for manufacturers in the Life Sciences industry, as defined by the BioPhorum Digital Plant Maturity Model.
Adaptable Landscape Components Facilitate Digital Transformation
The BioPhorum Operations Group (BPOG) established a digital plant maturity model (DPMM) for biopharmaceutical manufacturing to define stages of factory evolution “from simple paper-based plants through to the fully automated and integrated ‘adaptive plant’ of the future.” The model gives organizations a common tool for industry comparison and measurement of progress. It also provides a platform to facilitate collaboration within the life sciences industry and its technology providers.
BioPhorum MES of the Future
In BioPhorum’s MES of the Future Manifesto, Sean and Kate Porter collaborated with other biomanufacturing leaders on requirements for future Manufacturing Execution Systems.
Hygienic Pressure Measurement for Pharmaceutical and Bioprocess Applications
In a Process Instrumentation article, How to ensure reliable pressure transmitter measurement in pharmaceutical applications, Emerson’s Brandon Haschke shares how “Advanced hygienic pressure transmitters can reduce downtime and operating costs thanks to long-term stability that requires fewer calibrations.”
Overcoming Pharmaceutical and Biotech Manufacturing Challenges
Emerson’s Christian Berg presented Are We There Yet? Tackling Manufacturing Challenges with the Digital Plant at the Pharma Manufacturing World Summit in Boston.
Keep Up to Date With the Latest News and Updates
Follow Us
We invite you to follow us on Facebook, LinkedIn, Twitter and YouTube to stay up to date on the latest news, events and innovations that will help you face and solve your toughest challenges.
Do you want to reuse or translate content?
Just post a link to the entry and send us a quick note so we can share your work. Thank you very much.
Our Global Community
Emerson Exchange 365
The opinions expressed here are the personal opinions of the authors. Content published here is not read or approved by Emerson before it is posted and does not necessarily represent the views and opinions of Emerson.