Autologe und personalisierte Zell- und Gentherapien (CGTs) stehen an der Spitze der modernen Medizin und bieten potenzielle Heilungsmöglichkeiten für bisher unbehandelbare Krankheiten. Die Skalierung dieser Therapien stellt jedoch komplexe Herausforderungen dar, die sich von denen herkömmlicher Pharmazeutika unterscheiden. Die Herstellung erfordert die Verwaltung mehrerer kleiner, patientenspezifischer Chargen, was zu Problemen wie hohen Kosten, zeitkritischen Prozessen und behördlicher Kontrolle führt, sowie zu Anforderungen an präzises Datenmanagement und Rückverfolgbarkeit. Um diese Herausforderungen zu bewältigen, setzen Unternehmen digitale Strategien ein, die intelligente Automatisierung, Echtzeitüberwachung und KI-gesteuerte Prozessoptimierung umfassen. Das Whitepaper „Digital Excellence for Advanced Therapies“ von Emerson bietet wertvolle Einblicke in die digitale Transformation und zeigt Methoden zum Aufbau skalierbarer und konformer CGT-Produktionsumgebungen, zur Reduzierung von Chargenschwankungen und zur Beschleunigung der Auslieferung an die Patienten bei gleichzeitiger Gewährleistung der Einhaltung gesetzlicher Vorschriften.
cell and gene therapy
Liberar el potencial de la terapia celular y génica autóloga a través de la transformación digital
Las terapias celulares y génicas (TGC) autólogas y personalizadas están en la frontera de la medicina moderna y ofrecen posibles curas para enfermedades que antes eran intratables. Sin embargo, la ampliación de estas terapias presenta retos complejos distintos de los de los productos farmacéuticos tradicionales. La fabricación requiere la gestión de múltiples lotes pequeños y específicos para cada paciente, lo que conlleva problemas como costes elevados, procesos sensibles al tiempo y escrutinio normativo, junto con exigencias de gestión precisa de datos y trazabilidad. Para hacer frente a estos retos, las organizaciones están adoptando estrategias digitales que incluyen la automatización inteligente, la supervisión en tiempo real y la optimización de procesos impulsada por IA. El libro técnico de Emerson, «Digital Excellence for Advanced Therapies», proporciona información valiosa sobre la transformación digital, mostrando métodos para construir entornos de fabricación CGT escalables y conformes, reducir la variabilidad de los lotes y acelerar la entrega a los pacientes, garantizando el cumplimiento normativo efectivo.
Liberare il potenziale della terapia cellulare e genica autologa attraverso la trasformazione digitale
Le terapie cellulari e geniche autologhe e personalizzate (CGT) sono alla frontiera della medicina moderna e offrono potenziali cure per patologie precedentemente non trattabili. Tuttavia, la scalabilità di queste terapie presenta sfide complesse, diverse da quelle dei farmaci tradizionali. La produzione richiede la gestione di lotti multipli di piccole dimensioni e specifici per il paziente, con conseguenti problemi quali costi elevati, processi sensibili ai tempi e controlli normativi, oltre alla richiesta di una precisa gestione e tracciabilità dei dati. Per affrontare queste sfide, le organizzazioni stanno adottando strategie digitali che includono l’automazione intelligente, il monitoraggio in tempo reale e l’ottimizzazione dei processi guidata dall’intelligenza artificiale. Il white paper di Emerson, “Eccellenza digitale per le terapie avanzate”, fornisce preziose indicazioni sulla trasformazione digitale, illustrando i metodi per creare ambienti di produzione CGT scalabili e conformi, ridurre la variabilità dei lotti e accelerare la consegna ai pazienti, garantendo un’efficace conformità normativa.
Unlocking the Potential of Autologous Cell & Gene Therapy Through Digital Transformation
Autologous and personalized cell and gene therapies (CGTs) are at the frontier of modern medicine, offering potential cures for previously untreatable conditions. However, scaling these therapies presents complex challenges distinct from traditional pharmaceuticals. Manufacturing requires managing multiple small, patient-specific batches, leading to issues such as high costs, time-sensitive processes, and regulatory scrutiny, alongside demands for precise data management and traceability. To address these challenges, organizations are adopting digital strategies that include intelligent automation, real-time monitoring, and AI-driven process optimization. Emerson’s white paper, “Digital Excellence for Advanced Therapies,” provides valuable insights into digital transformation, showcasing methods to build scalable and compliant CGT manufacturing environments, reduce batch variability, and accelerate delivery to patients, ensuring effective regulatory compliance.
Smaller Batches Still Have Big Needs
There’s no question that today’s life sciences landscape is changing dramatically. As new personalized medicine solutions emerge, companies are rapidly changing scale to include more multi-product manufacturing facilities. Many of these facilities are manufacturing...
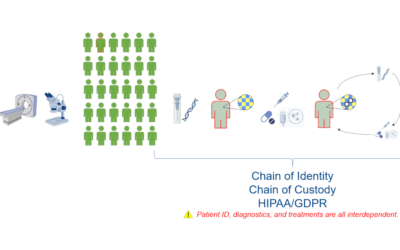
Traceability Across the Entire Drug Development Pipeline
Recently, as cell and gene therapies have begun to emerge in the life sciences manufacturing landscape, an important concept has begun to draw attention: traceability. However, it is important to remember that traceability was critical long before today’s most...
Manufacturing Challenges in Personalized Healthcare
In a Pharma Focus Asia webinar, Pursuing the Adaptive Plant to Enable Personalized Healthcare, Emerson’s Christian Berg provides a broader perspective of personalized healthcare and its challenges for the Life Sciences industry.
BioPhorum Cell and Gene Therapy Actors and Process Maps
Sean Buckley and co-authors from Roche, Takeda, and BioPhorum author CGT [cell & gene therapy] Actors and Process Maps which highlight six common types of CGT showing how the pattern of actors and process blocks varies by therapy type.
BioPhorum Cell and Gene Therapy Personas and User Stories
The BioPhorum CGT Personas and User Stories toolkit details the needs of all the key players involved in end-to-end cell and gene therapy (CGT) processes. It can be used by anyone who wishes to better understand how IT systems can support the manufacture and delivery of CGTs.
Making Medicine Personal with Flexible Automation Solutions
Personalized medicine manufacturers are rapidly changing their manufacturing platforms to provide a new scale of production. In the Emerson Exchange Virtual Series, life sciences experts held an open Q&A on the trajectory of cell and gene therapies, and how a...
Keep Up to Date With the Latest News and Updates
Follow Us
We invite you to follow us on Facebook, LinkedIn, Twitter and YouTube to stay up to date on the latest news, events and innovations that will help you face and solve your toughest challenges.
Do you want to reuse or translate content?
Just post a link to the entry and send us a quick note so we can share your work. Thank you very much.
Our Global Community
Emerson Exchange 365
The opinions expressed here are the personal opinions of the authors. Content published here is not read or approved by Emerson before it is posted and does not necessarily represent the views and opinions of Emerson.