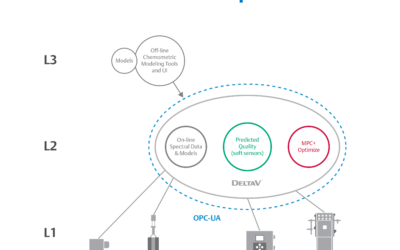
Traditional manufacturing practices have served the biopharmaceutical industry well for years, but times are changing and a need for speed to market in life sciences while reducing manufacturing costs is putting a focus on enabling real-time release (RTR)....
CGMP
Improve Speed to Market with Closed-Loop Process Control using Spectral PAT
We have witnessed an incredible change the last few years in how life sciences manufacturers deliver treatments. Today’s focus is on fast results, and that means products patients rely on cannot sit on shelves for weeks or months waiting on quality validation. In a...
Designing and Building Pharmaceutical and Biotech Smart Factories
Pharmaceutical and biotech manufacturers seek to improve both project and operational performance through implementation of smart factory technology. The smart factory fits right into the S95 enterprise automation model and provides out-of-box manufacturing...
Pharmaceutical and Biotech Manufacturing Data Integrity
The integrity of the data behind the production of pharmaceutical products is paramount. In recent years, the U.S. Food & Drug Administration (FDA) has increasingly observed data integrity related Current Good Manufacturing Practices (CGMP) violations during CGMP...
Pharmaceutical and Biotech Automation Knowledge Exchange
The pharmaceutical and biopharmaceutical industries have been interpreting recent FDA draft guidance—Data Integrity and Compliance With CGMP Guidance—and exploring new areas such as continuous manufacturing. The Emerson Life Sciences team has a full program of forums,...
What Do You Do for a Living?
Author: Michalle Adkins I often get asked, "So, what do you do for a living?" Boy is that a loaded question. When I say that I manage a group of Life Sciences Industry Consultants for Emerson—that really does not help to answer the question. Hmmm, how do I describe...
Optimizing Formulation, Fill and Finish Manufacturing Facilities
Pharmaceutical and biotech manufacturing processes are complex and require well integrated and documented flows of information from upfront forecast demand and customer orders through the production process to inventory receipt and order fulfillment. The components...
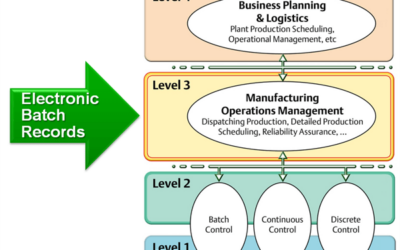
Electronic Batch Record Design Considerations
Last week, the 2nd International Summit on GMP, GCP & Quality Control was held in Chicago, Illinois USA. Emerson's Heather Schwalje, a senior Life Sciences consultant, presented Moving beyond part 11; Quality assurance considerations for translating Current Good...
Good Manufacturing Practice Background and Overview
Highly regulated industries, such as pharmaceutical and biotech manufacturing, face a myriad of compliance issues, which vary by region of the world and continuously involve. Emerson's Heather Schwalje presented Regulatory Drivers- GMPs 21st Century to Life Science...
Continuous Process Verification per FDA Process Validation Guidance
It was just about a year ago that the U.S. Food and Drug Administration published their Guidance for Industry - Process Validation: General Principles and Practices. I caught up with Emerson's Heather Schwalje, a senior consultant on the Life Sciences industry team....
Keep Up to Date With the Latest News and Updates
Follow Us
We invite you to follow us on Facebook, LinkedIn, Twitter and YouTube to stay up to date on the latest news, events and innovations that will help you face and solve your toughest challenges.
Do you want to reuse or translate content?
Just post a link to the entry and send us a quick note so we can share your work. Thank you very much.
Our Global Community
Emerson Exchange 365
The opinions expressed here are the personal opinions of the authors. Content published here is not read or approved by Emerson before it is posted and does not necessarily represent the views and opinions of Emerson.