For batch processes found in industries such as pharmaceutical & biopharmaceutical, food & beverage, specialty chemicals, etc., analytics technology advancements are helping to improve quality, consistency and reliability. I caught up with Emerson's Zuwei Jin...
continued process verification
Big Data and Process Analytics for Continued Process Verification
A biological drug is defined as a: …substance that is made from a living organism or its products and is used in the prevention, diagnosis, or treatment of cancer and other diseases. Biological drugs include antibodies, interleukins, and vaccines. The market for...
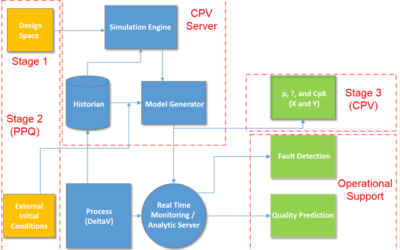
Continued Process Verification through Process Validation Stage 1, 2 and 3
For pharmaceutical and biotech manufacturers, Continued Process Verification is: ...the collection and analysis of end-to-end production components and processes data to ensure process is running under the state of control and product outputs are within predetermined...
Accurate and Repeatable Measurements in Pharmaceutical and Biotech Manufacturing
A Pharmaceutical Manufacturing magazine study revealed that pharmaceutical and biotech manufacturers were challenged to achieve batch after batch repeatability. I came across a recently published whitepaper, Consistency and Repeatability Through Accurate Measurements....
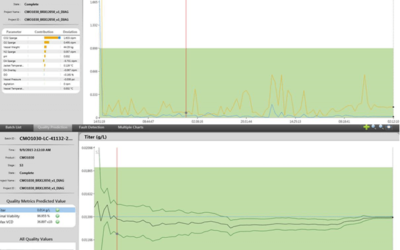
Online Multivariate Data Analysis for Continued Process Verification
Measuring quality in real-time improves the overall performance of a manufacturing process. The International Foundation Process Analytical Chemistry (IFPAC) is: …a world-wide, not-for-profit organization dedicated to the advancement of Process Analytical Technology...
US FDA Quality Metrics Guidelines Update
Author: Eric Kuebler Since the Continued Process Verification (CPV) guidance in 2011, the U.S. Food and Drug Administration (FDA) has been talking quality and verifying process state of control. Now the FDA is getting around to defining what that looks like and what...
Continued Process Verification in the Process Validation Lifecycle
In 2011, the U.S. Food and Drug Administration (FDA) issued a Guidance for Industry – Process Validation: General Principles and Practices. It highlighted a third validation stage goal of continued process verification (CPV) for: …continual assurance that the process...
Enabling Release by Exception Manufacturing
The Emerson Exchange conference October 6-10 in Orlando, Florida USA is rapidly approaching. I caught up with Emerson's Michalle Adkins who mentioned that her team of Life Sciences consultants would be highlighting the enablement of release by exception. Although...
Implementing Process Analytical Technology and Continuous Process Verification
In the 4:42 video, Life Science Drug Process Development and Manufacturing, Emerson's Gary Mitchell highlights the changes occurring for pharmaceutical and biotech manufacturers. Gary opens noting how these manufacturers are challenged to respond to new methods for...
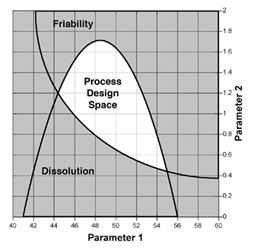
Implementing Quality by Design
Many manufacturers in the Life Sciences industry are challenged to respond to a new paradigm for process development and manufacturing. The FDA's cGMP for the 21st Century initiative is driving the industry to change its development and manufacturing to be based on...
Continuous Process Verification per FDA Process Validation Guidance
It was just about a year ago that the U.S. Food and Drug Administration published their Guidance for Industry - Process Validation: General Principles and Practices. I caught up with Emerson's Heather Schwalje, a senior consultant on the Life Sciences industry team....
Keep Up to Date With the Latest News and Updates
Follow Us
We invite you to follow us on Facebook, LinkedIn, Twitter and YouTube to stay up to date on the latest news, events and innovations that will help you face and solve your toughest challenges.
Do you want to reuse or translate content?
Just post a link to the entry and send us a quick note so we can share your work. Thank you very much.
Our Global Community
Emerson Exchange 365
The opinions expressed here are the personal opinions of the authors. Content published here is not read or approved by Emerson before it is posted and does not necessarily represent the views and opinions of Emerson.