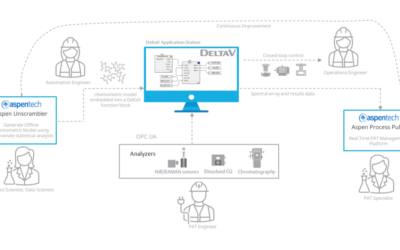
At the IFPAC-2023 meeting, Emerson’s Bruce Greenwald and Aspen Technology’s Geir Rune Flåten teamed together to present, Deploying a Rugged PAT Model Infrastructure for Real-Time Monitoring and Control.
pharmaceutical manufacturing
Unshackle Innovation with One Click Tech Transfer
Most people have heard the adage that, “COVID changed everything.” However, few people, when presented with that idea, think that any of those changes were positive. But we often forget that in the span of eight short months, the world saw a wide array of innovators...
Life Sciences Digital Maturity – Know Where You Are to Know Where You Can Go
To know where you’re going, you have to know where you are, and operating a life sciences manufacturing facility is no exception. Pharmaceutical processors regularly have to take stock of their technological capacity to keep a finger on the pulse of the four main...
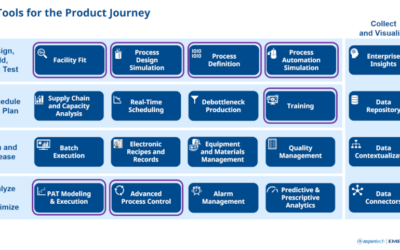
Digital Solutions for Process Development, Tech Transfer and Manufacturing
Emerson’s Michalle Adkins and AspenTech’s Chuck Miller team up to present Robust PAT Solutions to Support Process Development, Tech Transfer and Manufacturing at IFPAC 2023.
Traceability Across the Entire Drug Development Pipeline
Recently, as cell and gene therapies have begun to emerge in the life sciences manufacturing landscape, an important concept has begun to draw attention: traceability. However, it is important to remember that traceability was critical long before today’s most...
Better Treatment—For the Earth, and its People
It can be hard to keep up with the many rapid changes occurring in the life sciences industry these days. In an effort to embrace improved speed to market, sustainability, and quality, life sciences manufacturers are employing a wide range of software and technologies...
Four Pillars of Life Sciences Speed to Market
People are more aware than ever of the many amazing ways new treatments can improve their lives. As a result, life sciences companies are under more pressure than ever to safely deliver new, innovative treatments to market to satisfy customer demand. The key to...
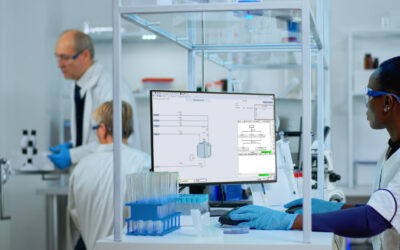
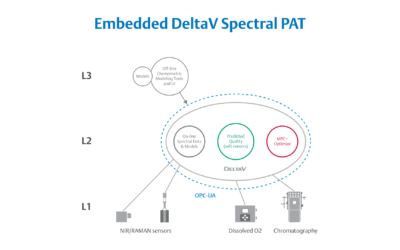
Embedded Spectral Process Analytic Technology
One of Pharma Manufacturing magazine’s Pharma Innovation Award winners is DeltaV Spectral Process Analytic Technology (PAT) which helps pharma and biopharma manufacturers build a foundation for better regulatory management while optimizing facility performance and throughput.
Improving Digital Maturity is Key to Life Sciences Success
In just the last 10 years, biopharmaceutical supply chains have changed dramatically. The single-source manufacturing model is becoming much rarer, making way for multi-enterprise manufacturing collaborations and partnerships to better meet the changing needs of the...
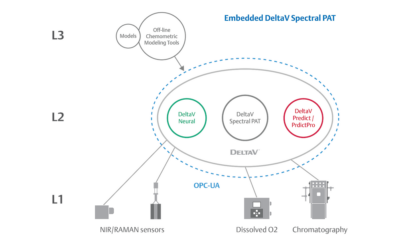
Real-World Implications of Spectral PAT for Life Sciences
Emerson offers an integrated Spectral PAT solution for DeltaV, allowing manufacturers to pursue closed-loop control (and real-time quality assessments) using in-line spectroscopic instruments.
Leverage Life Sciences Software to Reduce Time to Market
The days of maintaining paper records across the life sciences development and production pipeline are gone. Today’s regulatory requirements, not to mention the complexity of new treatments, requires organization that can only be provided electronically. However,...
Module Type Package Unlocks the Flexibility Driving Pharma Innovation
There can be little question that Industry 4.0 technologies are reshaping the way life sciences companies operate. In the wake of the rapid development of vaccines during the COVID-19 pandemic, more facilities than ever are being “born digital.” Some of these...
Keep Up to Date With the Latest News and Updates
Follow Us
We invite you to follow us on Facebook, LinkedIn, Twitter and YouTube to stay up to date on the latest news, events and innovations that will help you face and solve your toughest challenges.
Do you want to reuse or translate content?
Just post a link to the entry and send us a quick note so we can share your work. Thank you very much.
Our Global Community
Emerson Exchange 365
The opinions expressed here are the personal opinions of the authors. Content published here is not read or approved by Emerson before it is posted and does not necessarily represent the views and opinions of Emerson.