In this podcast, Emerson’s Jorge Costa and Bruce Greenwald join Jim Cahill to discuss the application of PAT technologies not only within the Life Sciences but across many of the process and hybrid manufacturing industries.
Process Analytical Technology
Prototype Sensing Technologies are at the Forefront of Innovation
On June 4-7, experts in process analytical technology (PAT) gathered for the 2023 IFPAC conference. While there, they discussed the latest developments in PAT, quality by design, and overall process monitoring and control within the pharmaceutical, biotechnology,...
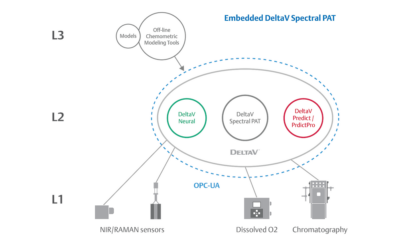
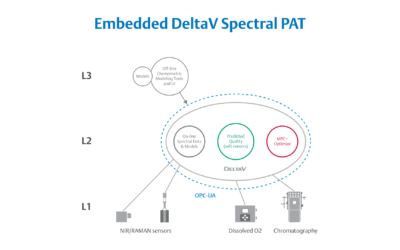
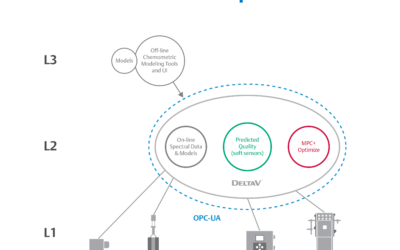
Embedded Spectral Process Analytic Technology
One of Pharma Manufacturing magazine’s Pharma Innovation Award winners is DeltaV Spectral Process Analytic Technology (PAT) which helps pharma and biopharma manufacturers build a foundation for better regulatory management while optimizing facility performance and throughput.
Real-World Implications of Spectral PAT for Life Sciences
Emerson offers an integrated Spectral PAT solution for DeltaV, allowing manufacturers to pursue closed-loop control (and real-time quality assessments) using in-line spectroscopic instruments.
PAT in the Chemical Industry: The Effectiveness of Analyzers
In a PI Magazine article, Emerson’s Charu Pandey considers how the chemical processing industry is re-learning critical technology lessons.
BioPhorum’s Roadmap for Speed to Market with In-Line Monitoring and Real-Time Release
Traditional manufacturing practices have served the biopharmaceutical industry well for years, but times are changing and a need for speed to market in life sciences while reducing manufacturing costs is putting a focus on enabling real-time release (RTR)....
Improve Speed to Market with Closed-Loop Process Control using Spectral PAT
We have witnessed an incredible change the last few years in how life sciences manufacturers deliver treatments. Today’s focus is on fast results, and that means products patients rely on cannot sit on shelves for weeks or months waiting on quality validation. In a...
Successfully Implementing Process Analytical Technology
It's been well more than a decade since the U.S. Food & Drug Administration (FDA) announced a Process Analytical Technology (PAT) approach for pharmaceutical manufacturers in their Guidance for Industry PAT — A Framework for Innovative Pharmaceutical Development,...
Trends in the Life Sciences Industry
For pharmaceutical and biotech manufacturers, trends in the market place are driving changes in the way they have historically operated their production processes. I caught up with Emerson's Michalle Adkins who shared these trends with me. She identified six major...
Accurate and Repeatable Measurements in Pharmaceutical and Biotech Manufacturing
A Pharmaceutical Manufacturing magazine study revealed that pharmaceutical and biotech manufacturers were challenged to achieve batch after batch repeatability. I came across a recently published whitepaper, Consistency and Repeatability Through Accurate Measurements....
Is Process Analytical Technology Rocket Science?
More than a decade ago, the U.S. Food and Drug Administration published, Guidance for Industry PAT — A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance. In this document, the FDA explained: The scientific, risk-based framework...
Online Multivariate Data Analysis for Continued Process Verification
Measuring quality in real-time improves the overall performance of a manufacturing process. The International Foundation Process Analytical Chemistry (IFPAC) is: …a world-wide, not-for-profit organization dedicated to the advancement of Process Analytical Technology...
Keep Up to Date With the Latest News and Updates
Follow Us
We invite you to follow us on Facebook, LinkedIn, Twitter and YouTube to stay up to date on the latest news, events and innovations that will help you face and solve your toughest challenges.
Do you want to reuse or translate content?
Just post a link to the entry and send us a quick note so we can share your work. Thank you very much.
Our Global Community
Emerson Exchange 365
The opinions expressed here are the personal opinions of the authors. Content published here is not read or approved by Emerson before it is posted and does not necessarily represent the views and opinions of Emerson.