Digital twins provide a simulation of the manufacturing or production process as well as the automation systems which control it. Depending on the level of fidelity of the process models, this digital twin can have many uses. I caught up with Emerson's Zuwei Jin who...
Zuwei Jin
Manufacturing Execution Systems in Drug Research and Development
The U.S. Food & Drug Administration (FDA) outlines the drug development process in five steps: Discovery and development Preclinical research Clinical trials FDA review FDA post-market safety monitoring I caught up with Emerson's Zuwei Jin whom you may recall from...
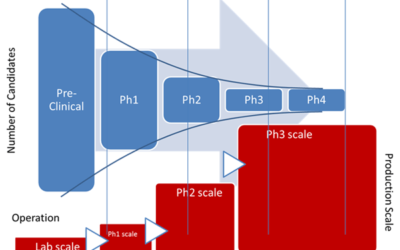
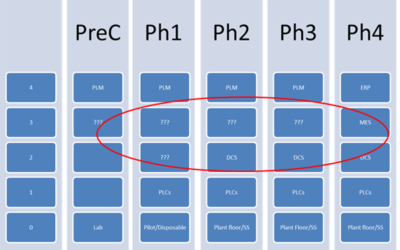
Tech Transfer in Drug Development Pipeline and Industry 4.0
Author: Zuwei Jin Among all of the important trends in life sciences industry such as modular plants, integrated modules, out-of-box smart factory, single-use equipment, PAT, and manufacturing execution systems (MES), IIOT has been receiving high interest including...
Designing and Building Pharmaceutical and Biotech Smart Factories
Pharmaceutical and biotech manufacturers seek to improve both project and operational performance through implementation of smart factory technology. The smart factory fits right into the S95 enterprise automation model and provides out-of-box manufacturing...
Integrating Analytics Across Process Development Lifecycle
For batch processes found in industries such as pharmaceutical & biopharmaceutical, food & beverage, specialty chemicals, etc., analytics technology advancements are helping to improve quality, consistency and reliability. I caught up with Emerson's Zuwei Jin...
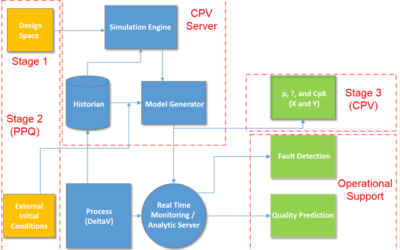
Big Data and Process Analytics for Continued Process Verification
A biological drug is defined as a: …substance that is made from a living organism or its products and is used in the prevention, diagnosis, or treatment of cancer and other diseases. Biological drugs include antibodies, interleukins, and vaccines. The market for...
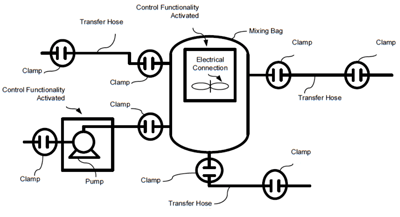
Integrating Single Use Equipment More Easily with Control and Manufacturing Execution Systems
One of the trends in the biopharmaceutical industries that have taken root over the past decade and a half is the use of single-use, disposable equipment in the manufacturing process. Prior to their use, these facilities relied on inflexible, hard-piped equipment. An...
Continued Process Verification through Process Validation Stage 1, 2 and 3
For pharmaceutical and biotech manufacturers, Continued Process Verification is: ...the collection and analysis of end-to-end production components and processes data to ensure process is running under the state of control and product outputs are within predetermined...
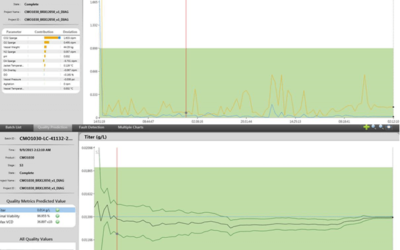
Online Multivariate Data Analysis for Continued Process Verification
Measuring quality in real-time improves the overall performance of a manufacturing process. The International Foundation Process Analytical Chemistry (IFPAC) is: …a world-wide, not-for-profit organization dedicated to the advancement of Process Analytical Technology...
Clarifying Design Space, Process Analytical Technology and Quality by Design
For pharmaceutical and biopharmaceutical manufacturers, there are a lot confusion around the concepts of design space (DS), Process Analytical Technology (PAT), and Quality by Design (QbD). Emerson's Zuwei Jin believes that this confusion has largely limited the...
Continued Process Verification in the Process Validation Lifecycle
In 2011, the U.S. Food and Drug Administration (FDA) issued a Guidance for Industry – Process Validation: General Principles and Practices. It highlighted a third validation stage goal of continued process verification (CPV) for: …continual assurance that the process...
Improving Technology Transfer by Earlier Adoption of Standards and Software Platforms
In 2011, the U.S. Food & Drug Administration (FDA) published their Guidance for Industry – Process Validation: General Principles and Practices. A PharmManufacturing.com article, A Framework for Technology Transfer to Satisfy the Requirements of the New Process...
Keep Up to Date With the Latest News and Updates
Follow Us
We invite you to follow us on Facebook, LinkedIn, Twitter and YouTube to stay up to date on the latest news, events and innovations that will help you face and solve your toughest challenges.
Do you want to reuse or translate content?
Just post a link to the entry and send us a quick note so we can share your work. Thank you very much.
Our Global Community
Emerson Exchange 365
The opinions expressed here are the personal opinions of the authors. Content published here is not read or approved by Emerson before it is posted and does not necessarily represent the views and opinions of Emerson.